W Doping in Ni12P5 as a Platform to Enhance Overall Electrochemical Water Splitting
- PMID: 34963045
- PMCID: PMC8762645
- DOI: 10.1021/acsami.1c16755
W Doping in Ni12P5 as a Platform to Enhance Overall Electrochemical Water Splitting
Abstract
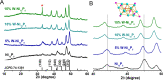
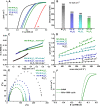
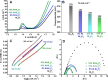
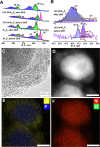
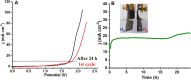
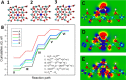
Bifunctional electrocatalysts for efficient hydrogen generation from water splitting must overcome both the sluggish water dissociation step of the alkaline hydrogen evolution half-reaction (HER) and the kinetic barrier of the anodic oxygen evolution half-reaction (OER). Nickel phosphides are a promising catalysts family and are known to develop a thin active layer of oxidized Ni in an alkaline medium. Here, Ni12P5 was recognized as a suitable platform for the electrochemical production of γ-NiOOH─a particularly active phase─because of its matching crystallographic structure. The incorporation of tungsten by doping produces additional surface roughness, increases the electrochemical surface area (ESCA), and reduces the energy barrier for electron-coupled water dissociation (the Volmer step for the formation of Hads). When serving as both the anode and cathode, the 15% W-Ni12P5 catalyst provides an overall water splitting current density of 10 mA cm-2 at a cell voltage of only 1.73 V with good durability, making it a promising bifunctional catalyst for practical water electrolysis.
Keywords: DFT calculations; nickel phosphide; oxygen evolution reaction; structure-function relationship; γ-NiOOH.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
LinkOut - more resources
Full Text Sources

