Association of entorhinal cortical tau deposition and hippocampal synaptic density in older individuals with normal cognition and early Alzheimer's disease
- PMID: 34963063
- PMCID: PMC8761170
- DOI: 10.1016/j.neurobiolaging.2021.11.004
Association of entorhinal cortical tau deposition and hippocampal synaptic density in older individuals with normal cognition and early Alzheimer's disease
Abstract
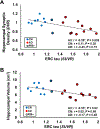
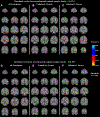
Sites of early neuropathologic change provide important clues regarding the initial clinical features of Alzheimer's disease (AD). We have shown significant reductions in hippocampal synaptic density in participants with AD, consistent with the early degeneration of entorhinal cortical (ERC) cells that project to hippocampus via the perforant path. In this study, [11C]UCB-J binding to synaptic vesicle glycoprotein 2A (SV2A) and [18F]flortaucipir binding to tau were measured via PET in 10 participants with AD (5 mild cognitive impairment, 5 mild dementia) and 10 cognitively normal participants. In the overall sample, ERC tau was inversely associated with hippocampal synaptic density (r = -0.59, p = 0.009). After correction for partial volume effects, the association of ERC tau with hippocampal synaptic density was stronger in the overall sample (r = -0.61, p = 0.007) and in the AD group where the effect size was large, but not statistically significant (r = -0.58, p = 0.06). This inverse association of ERC tau and hippocampal synaptic density may reflect synaptic failure due to tau pathology in ERC neurons projecting to the hippocampus.
Keywords: Alzheimer's disease; PET; SV2A; Synaptic density; Tau; [(11)C]UCB-J; [(18)F]Flortaucipir.
Copyright © 2021. Published by Elsevier Inc.
Figures


References
-
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH 2011. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7(3), 270–9. doi:10.1016/j.jalz.2011.03.008. - DOI - PMC - PubMed
-
- Bastin C, Bahri MA, Meyer F, Manard M, Delhaye E, Plenevaux A, Becker G, Seret A, Mella C, Giacomelli F, Degueldre C, Balteau E, Luxen A, Salmon E 2019. In vivo imaging of synaptic loss in Alzheimer’s disease with [18F]UCB-H positron emission tomography. Eur J Nucl Med Mol Imaging. doi:10.1007/s00259-019-04461-x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

