Novel brain-targeted nanomicelles for anti-glioma therapy mediated by the ApoE-enriched protein corona in vivo
- PMID: 34963449
- PMCID: PMC8715648
- DOI: 10.1186/s12951-021-01097-8
Novel brain-targeted nanomicelles for anti-glioma therapy mediated by the ApoE-enriched protein corona in vivo
Abstract
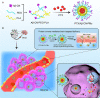
Background: The interactions between nanoparticles (NPs) and plasma proteins form a protein corona around NPs after entering the biological environment, which provides new biological properties to NPs and mediates their interactions with cells and biological barriers. Given the inevitable interactions, we regard nanoparticle‒protein interactions as a tool for designing protein corona-mediated drug delivery systems. Herein, we demonstrate the successful application of protein corona-mediated brain-targeted nanomicelles in the treatment of glioma, loading them with paclitaxel (PTX), and decorating them with amyloid β-protein (Aβ)-CN peptide (PTX/Aβ-CN-PMs). Aβ-CN peptide, like the Aβ1-42 peptide, specifically binds to the lipid-binding domain of apolipoprotein E (ApoE) in vivo to form the ApoE-enriched protein corona surrounding Aβ-CN-PMs (ApoE/PTX/Aβ-CN-PMs). The receptor-binding domain of the ApoE then combines with low-density lipoprotein receptor (LDLr) and LDLr-related protein 1 receptor (LRP1r) expressed in the blood-brain barrier and glioma, effectively mediating brain-targeted delivery.
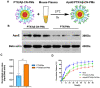
Methods: PTX/Aβ-CN-PMs were prepared using a film hydration method with sonication, which was simple and feasible. The specific formation of the ApoE-enriched protein corona around nanoparticles was characterized by Western blotting analysis and LC-MS/MS. The in vitro physicochemical properties and in vivo anti-glioma effects of PTX/Aβ-CN-PMs were also well studied.
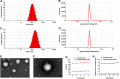
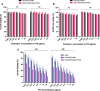
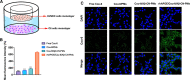
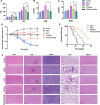
Results: The average size and zeta potential of PTX/Aβ-CN-PMs and ApoE/PTX/Aβ-CN-PMs were 103.1 nm, 172.3 nm, 7.23 mV, and 0.715 mV, respectively. PTX was efficiently loaded into PTX/Aβ-CN-PMs, and the PTX release from rhApoE/PTX/Aβ-CN-PMs exhibited a sustained-release pattern in vitro. The formation of the ApoE-enriched protein corona significantly improved the cellular uptake of Aβ-CN-PMs on C6 cells and human umbilical vein endothelial cells (HUVECs) and enhanced permeability to the blood-brain tumor barrier in vitro. Meanwhile, PTX/Aβ-CN-PMs with ApoE-enriched protein corona had a greater ability to inhibit cell proliferation and induce cell apoptosis than taxol. Importantly, PTX/Aβ-CN-PMs exhibited better anti-glioma effects and tissue distribution profile with rapid accumulation in glioma tissues in vivo and prolonged median survival of glioma-bearing mice compared to those associated with PMs without the ApoE protein corona.
Conclusions: The designed PTX/Aβ-CN-PMs exhibited significantly enhanced anti-glioma efficacy. Importantly, this study provided a strategy for the rational design of a protein corona-based brain-targeted drug delivery system. More crucially, we utilized the unfavorable side of the protein corona and converted it into an advantage to achieve brain-targeted drug delivery.
Keywords: ApoE protein corona; Glioma; Paclitaxel; Targeting therapy.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest in the paper.
Figures







References
-
- Anjum K, Shagufta BI, Abbas SQ, Patel S, Khan I, Shah S, et al. Current status and future therapeutic perspectives of glioblastoma multiforme (GBM) therapy: A review. Biomed Pharmacother. 2017;92:681–689. - PubMed
-
- Chen Z, Zhai M, Xie X, Zhang Y, Ma S, Li Z, et al. Apoferritin nanocage for brain targeted doxorubicin delivery. Mol Pharm. 2017;14(9):3087–3097. - PubMed
-
- Ruan H, Chai Z, Shen Q, Chen X, Su B, Xie C, et al. A novel peptide ligand RAP12 of LRP1 for glioma targeted drug delivery. J Control Release. 2018;279:306–315. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

