Immunoprofiling of active and inactive systemic juvenile idiopathic arthritis reveals distinct biomarkers: a single-center study
- PMID: 34963488
- PMCID: PMC8713412
- DOI: 10.1186/s12969-021-00660-9
Immunoprofiling of active and inactive systemic juvenile idiopathic arthritis reveals distinct biomarkers: a single-center study
Abstract
Background: This study aimed to perform an immunoprofiling of systemic juvenile idiopathic arthritis (sJIA) in order to define biomarkers of clinical use as well as reveal new immune mechanisms.
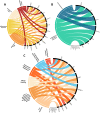
Methods: Immunoprofiling of plasma samples from a clinically well-described cohort consisting of 21 sJIA patients as well as 60 age and sex matched healthy controls, was performed by a highly sensitive proteomic immunoassay. Based on the biomarkers being significantly up- or down-regulated in cross-sectional and paired analysis, related canonical pathways and cellular functions were explored by Ingenuity Pathway Analysis (IPA).
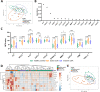
Results: The well-studied sJIA biomarkers, IL6, IL18 and S100A12, were confirmed to be increased during active sJIA as compared to healthy controls. IL18 was the only factor found to be increased during inactive sJIA as compared to healthy controls. Novel factors, including CASP8, CCL23, CD6, CXCL1, CXCL11, CXCL5, EIF4EBP1, KITLG, MMP1, OSM, SIRT2, SULT1A1 and TNFSF11, were found to be differentially expressed in active and/or inactive sJIA and healthy controls. No significant pathway activation could be predicted based on the limited factor input to the IPA. High Mobility Group Box 1 (HMGB1), a damage associated molecular pattern being involved in a series of inflammatory diseases, was determined to be higher in active sJIA than inactive sJIA.
Conclusions: We could identify a novel set of biomarkers distinguishing active sJIA from inactive sJIA or healthy controls. Our findings enable a better understanding of the immune mechanisms active in sJIA and aid the development of future diagnostic and therapeutic strategies.
Keywords: Cytokines and inflammatory mediators; High mobility group Box 1; Inflammation; Ingenuity pathway analysis; Proteomics; Systemic juvenile idiopathic arthritis.
© 2021. The Author(s).
Conflict of interest statement
The authors have declared no competing interests.
Figures



References
-
- Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, He X, Maldonado-Cocco J, Orozco-Alcala J, Prieur AM, Suarez-Almazor ME, Woo P, International League of Associations for Rheumatology International league of associations for rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–392. - PubMed
-
- Quartier P, Allantaz F, Cimaz R, Pillet P, Messiaen C, Bardin C, Bossuyt X, Boutten A, Bienvenu J, Duquesne A, Richer O, Chaussabel D, Mogenet A, Banchereau J, Treluyer JM, Landais P, Pascual V. A multicentre, randomised, double-blind, placebo-controlled trial with the Interleukin-1 receptor antagonist Anakinra in patients with systemic-onset juvenile idiopathic arthritis (Anajis trial) Ann Rheum Dis. 2011;70(5):747–754. doi: 10.1136/ard.2010.134254. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

