Assessment of the control measures of the category A diseases of Animal Health Law: sheep and goat pox
- PMID: 34963791
- PMCID: PMC8711069
- DOI: 10.2903/j.efsa.2021.6933
Assessment of the control measures of the category A diseases of Animal Health Law: sheep and goat pox
Abstract
EFSA received a mandate from the European Commission to assess the effectiveness of some of the control measures against diseases included in the Category A list according to Regulation (EU) 2016/429 on transmissible animal diseases ('Animal Health Law'). This opinion belongs to a series of opinions where these control measures will be assessed, with this opinion covering the assessment of control measures for sheep and goat pox. In this opinion, EFSA and the AHAW Panel of experts review the effectiveness of: (i) clinical and laboratory sampling procedures, (ii) monitoring period and (iii) the minimum radii of the protection and surveillance zones, and the minimum length of time the measures should be applied in these zones. The general methodology used for this series of opinions has been published elsewhere; nonetheless, the transmission kernels used for the assessment of the minimum radii of the protection and surveillance zones are shown. Several scenarios for which these control measures had to be assessed were designed and agreed prior to the start of the assessment. Different risk-based sampling procedures based on clinical visits and laboratory testing are assessed in case of outbreak suspicion, granting animal movements and for repopulation purposes. The monitoring period of 21 days was assessed as effective. The estimated probability of transmission beyond the protection zone of 3 km radius from an infectious establishment is 9.6% (95% CI: 3.1-25.8%) and 2.3% (95% CI: 1-5.5%) for the surveillance zone of 10 km radius. This may be considered sufficient to contain the disease spread (95% probability of containing transmission corresponds to 5.3 Km). To contain 99% of the spread, the radius should be increased to 19.4 km (95% CI: 9.8-26.8). This may increase the number of farms in the surveillance zone, since the area would increase fourfold.
Keywords: SPP/GTP; monitoring period; protection zone; sampling procedures; surveillance zone.
© 2021 European Food Safety Authority. EFSA Journal published by John Wiley and Sons Ltd on behalf of European Food Safety Authority.
Figures
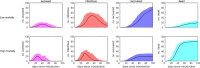
The plots show the median (solid line) and 95% prediction interval (shading) for the number of exposed animals (first column; magenta), infectious animals (second column; red), recovered animals (third column; blue) and cumulative number of dead animals (fourth column; cyan) for four scenarios under different mortality rates in a flock of 100 animals (rows; see Table 2 for details).
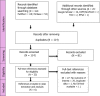
Parameters were estimated by fitting the models to data on a sheep pox epidemic in the Evros region of Greece, 2013–2015.
Similar articles
-
Assessment of the control measures of the category A diseases of Animal Health Law: Rift Valley Fever.EFSA J. 2022 Jan 19;20(1):e07070. doi: 10.2903/j.efsa.2022.7070. eCollection 2022 Jan. EFSA J. 2022. PMID: 35079289 Free PMC article.
-
Assessment of the control measures of the category A diseases of Animal Health Law: peste des petits ruminants.EFSA J. 2021 Jul 30;19(7):e06708. doi: 10.2903/j.efsa.2021.6708. eCollection 2021 Jul. EFSA J. 2021. PMID: 34354766 Free PMC article.
-
Assessment of the control measures of the category A diseases of Animal Health Law: Classical Swine Fever.EFSA J. 2021 Jul 21;19(7):e06707. doi: 10.2903/j.efsa.2021.6707. eCollection 2021 Jul. EFSA J. 2021. PMID: 34306220 Free PMC article.
-
[Single-donor (apheresis) platelets and pooled whole-blood-derived platelets--significance and assessment of both blood products].Clin Lab. 2014;60(4):S1-39. doi: 10.7754/clin.lab.2014.140210. Clin Lab. 2014. PMID: 24779310 Review. German.
-
International travel-related control measures to contain the COVID-19 pandemic: a rapid review.Cochrane Database Syst Rev. 2021 Mar 25;3(3):CD013717. doi: 10.1002/14651858.CD013717.pub2. Cochrane Database Syst Rev. 2021. PMID: 33763851 Free PMC article.
Cited by
-
Spatio-temporal analysis of sheep and goat pox outbreaks in Uganda during 2011-2022.BMC Vet Res. 2023 Oct 28;19(1):224. doi: 10.1186/s12917-023-03788-w. BMC Vet Res. 2023. PMID: 37891597 Free PMC article.
-
Assessment of the control measures of the Category A diseases of the Animal Health Law: prohibitions in restricted zones and risk-mitigating treatments for products of animal origin and other materials.EFSA J. 2022 Aug 9;20(8):e07443. doi: 10.2903/j.efsa.2022.7443. eCollection 2022 Aug. EFSA J. 2022. PMID: 35958104 Free PMC article.
References
-
- Anderson D and Watson R, 1980. On the spread of a disease with gamma distributed latent and infectious periods. Biometrika, 67, 191–198.
-
- Ayalet G, Fasil N, Jembere S, Mekonen G, Sori T and Negussie H, 2012. Study on immunogenicity of combined sheep and goat pox and peste des petitis ruminants vaccines in small ruminants in Ethiopia. African Journal of Microbiology Research, 6, 7212–7217.
-
- Babiuk S, Wallace D, Smith S, Bowden T, Dalman B, Parkyn G, Copps J and Boyle D, 2009. Detection of antibodies against capripoxviruses using an inactivated sheeppox virus ELISA. Transboundary and Emerging Diseases, 56, 132–141. - PubMed
-
- Bhanuprakash V, Indrani BK, Hosamani M and Singh RK, 2006. The current status of sheep pox disease. Comparative immunology, microbiology and infectious diseases, 29, 27–60. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
