Ultrasound-Controlled CRISPR/Cas9 System Augments Sonodynamic Therapy of Hepatocellular Carcinoma
- PMID: 34963897
- PMCID: PMC8704033
- DOI: 10.1021/acscentsci.1c01143
Ultrasound-Controlled CRISPR/Cas9 System Augments Sonodynamic Therapy of Hepatocellular Carcinoma
Abstract
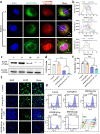
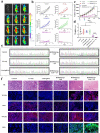
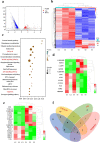
Sonodynamic therapy (SDT), relying on the generation of reactive oxygen species (ROS), is a promising clinical therapeutic modality for the treatment of hepatocellular carcinoma (HCC) due to its noninvasiveness and high tissue-penetration depth, whereas the oxidative stress and antioxidative defense system in cancer cells significantly restrict the prevalence of SDT. Herein, we initially identified that NFE2L2 was immediately activated during SDT, which further inhibited SDT efficacy. To address this intractable issue, an ultrasound remote control of the cluster regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated protein 9 (Cas9) release system (HMME@Lip-Cas9) was meticulously designed and constructed, which precisely knocks down NFE2L2 to alleviate the adverse effects and augment the therapeutic efficiency of SDT. The hematoporphyrin monomethyl ether (HMME) in this system yielded abundant ROS to damage cancer cells under ultrasound irradiation, and meanwhile the generated ROS could induce lysosomal rupture to release Cas9/single guide RNA ribonucleoprotein (RNP) and destroy the oxidative stress-defensing system, significantly promoting tumor cell apoptosis. This study provides a new paradigm for HCC management and lays the foundation for the widespread application of CRISPR/Cas9 with promising clinical translation, meanwhile developing a synergistic therapeutic modality in the combination of SDT with gene editing.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Bruix J.; Takayama T.; Mazzaferro V.; Chau G. Y.; Yang J.; Kudo M.; Cai J.; Poon R. T.; Han K. H.; Tak W. Y.; et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015, 16 (13), 1344–54. 10.1016/S1470-2045(15)00198-9. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

