Axonics Sacral Neuromodulation System for Treating Refractory Overactive Bladder: A NICE Medical Technologies Guidance
- PMID: 34964090
- PMCID: PMC9021055
- DOI: 10.1007/s40258-021-00701-0
Axonics Sacral Neuromodulation System for Treating Refractory Overactive Bladder: A NICE Medical Technologies Guidance
Abstract
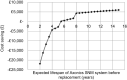
The Axonics sacral neuromodulation (SNM) system can be used by people with refractory overactive bladder (OAB) to reduce symptoms of urge urinary incontinence and urinary frequency, where conservative treatments have failed or are not suitable. It is the first system for this indication that makes use of a rechargeable battery to prolong the lifespan of the implanted device, with the potential advantage of reducing the frequency of surgical replacement procedures and associated complications. We describe the evidence considered by the UK National Institute of Health and Care Excellence (NICE) in their evaluation of this evidence, supported by Cedar Healthcare Technology Research Centre. Two observational studies provided descriptive data that suggested improvement in control of symptoms after implantation of the Axonics SNM system; however, there was no peer-reviewed evidence that directly compared rechargeable and non-rechargeable SNM systems. In the absence of long-term data, economic modelling relies on the accuracy of battery life estimates. The evidence supports the case for adopting the Axonics SNM system for treating refractory OAB, when conservative treatment or treatment with medicines has not worked. This conclusion is consistent with other relevant NICE guidelines. Use of Axonics SNM technology in the UK National Health Service (NHS) is associated with a potential cost saving of £6025 per person over a 15-year period when compared with an equivalent non-rechargeable SNM system, assuming the claimed battery life estimate (a minimum of 15 years) is accurate. The cost savings are estimated to start around 6 years after implantation.
© 2021. The Author(s).
Conflict of interest statement
RLP, MD and RM are NHS employees, which has a financial interest in the guidance on which this project is based. HM is a Cardiff University employee and has no conflicts of interest to declare. RB and TO are NICE employees and had no role in the production of the assessment report but contributed to the preparation of this manuscript. This summary of the Medical Technology Guidance was produced following publication of the final guidance report. The article has not been externally peer reviewed by Applied Health Economics and Health Policy but has been reviewed externally by NICE. Cedar was funded by the NICE MTEP for their work.
Figures
Comment in
-
Voiding Function and Dysfunction, Bladder Physiology and Pharmacology, and Female Urology.J Urol. 2022 Jun;207(6):1352-1356. doi: 10.1097/JU.0000000000002662. Epub 2022 Mar 24. J Urol. 2022. PMID: 35321553 No abstract available.
References
-
- National Institute for Health and Care Excellence. Medical technologies guidance. London: NICE; 2021. Available at: https://www.nice.org.uk/About/What-we-do/Our-Programmes/NICE-guidance/NI.... Accessed 14 May 2021.
-
- Poole RL, Dale M, Morgan H, Ryczek E, Cohen B, Carolan-Rees G. Axonics sacral neuromodulation system for treating refractory overactive bladder (external assessment report). 2019. Available at: https://www.nice.org.uk/guidance/mtg50/documents/supporting-documentation-2. Accessed 21 Mar 2021.
-
- National Institute for Health and Care Excellence. Axonics sacral neuromodulation system for treating refractory overactive bladder. London: NICE; Sep 2020. (Medical technologies guidance [MTG50]). Available at: https://www.nice.org.uk/guidance/mtg50. Accessed 21 Mar 2021.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical