First-in-Human Experience and Acute Procedural Outcomes Using a Novel Pulsed Field Ablation System: The PULSED AF Pilot Trial
- PMID: 34964367
- PMCID: PMC8772438
- DOI: 10.1161/CIRCEP.121.010168
First-in-Human Experience and Acute Procedural Outcomes Using a Novel Pulsed Field Ablation System: The PULSED AF Pilot Trial
Abstract
Background: Pulsed field ablation (PFA) is a novel form of ablation using electrical fields to ablate cardiac tissue. There are only limited data assessing the feasibility and safety of this type of ablation in humans.
Methods: PULSED AF (Pulsed Field Ablation to Irreversibly Electroporate Tissue and Treat AF; https://www.clinicaltrials.gov; unique identifier: NCT04198701) is a nonrandomized, prospective, multicenter, global, premarket clinical study. The first-in-human pilot phase evaluated the feasibility and efficacy of pulmonary vein isolation using a novel PFA system delivering bipolar, biphasic electrical fields through a circular multielectrode array catheter (PulseSelect; Medtronic, Inc). Thirty-eight patients with paroxysmal or persistent atrial fibrillation were treated in 6 centers in Australia, Canada, the United States, and the Netherlands. The primary outcomes were ability to achieve acute pulmonary vein isolation intraprocedurally and safety at 30 days.
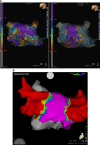
Results: Acute electrical isolation was achieved in 100% of pulmonary veins (n=152) in the 38 patients. Skin-to-skin procedure time was 160±91 minutes, left atrial dwell time was 82±35 minutes, and fluoroscopy time was 28±9 minutes. No serious adverse events related to the PFA system occurred in the 30-day follow-up including phrenic nerve injury, esophageal injury, stroke, or death.
Conclusions: In this first-in-human clinical study, 100% pulmonary vein isolation was achieved using only PFA with no PFA system-related serious adverse events. Graphic Abstract: A graphic abstract is available for this article.
Keywords: atrial fibrillation; catheter ablation; electroporation; follow-up studies.
Figures


References
-
- Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–e444. doi: 10.1016/j.hrthm.2017.05.012 - PMC - PubMed
-
- Muthalaly RG, John RM, Schaeffer B, Tanigawa S, Nakamura T, Kapur S, Zei PC, Epstein LM, Tedrow UB, Michaud GF, et al. Temporal trends in safety and complication rates of catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2018;29:854–860. doi: 10.1111/jce.13484 - PubMed
-
- Kotnik T, Rems L, Tarek M, Miklavčič D. Membrane electroporation and electropermeabilization: mechanisms and models. Annu Rev Biophys. 2019;48:63–91. doi: 10.1146/annurev-biophys-052118-115451 - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

