Population Pharmacokinetics and Exposure-Response Relationships of Astegolimab in Patients With Severe Asthma
- PMID: 34964491
- PMCID: PMC9303772
- DOI: 10.1002/jcph.2021
Population Pharmacokinetics and Exposure-Response Relationships of Astegolimab in Patients With Severe Asthma
Abstract
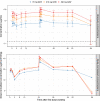
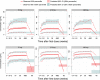
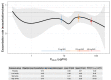
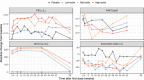
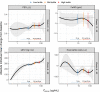
Astegolimab is a fully human immunoglobulin G2 monoclonal antibody that binds to the ST2 receptor and blocks the interleukin-33 signaling. It was evaluated in patients with uncontrolled severe asthma in the phase 2b study (Zenyatta) at doses of 70, 210, and 490 mg subcutaneously every 4 weeks for 52 weeks. This work aimed to characterize astegolimab pharmacokinetics, identify influential covariates contributing to its interindividual variability, and make a descriptive assessment of the exposure-response relationships. A population pharmacokinetic model was developed using data from 368 patients in the Zenyatta study. Predicted average steady-state concentration was used in the subsequent exposure-response analyses, which evaluated efficacy (asthma exacerbation rate) and biomarker end points including forced expiratory volume in 1 second, fraction exhaled nitric oxide, blood eosinophils, and soluble ST2. A 2-compartment disposition model with first-order elimination and first-order absorption best described the astegolimab pharmacokinetics. The relative bioavailability for the 70-mg dose was 15.3% lower. Baseline body weight, estimated glomerular filtration rate, and eosinophils were statistically correlated with pharmacokinetic parameters, but only body weight had a clinically meaningful influence on the steady-state exposure (ratios exceeding 0.8-1.25). The exposure-response of efficacy and biomarkers were generally flat with a weak trend in favor of the highest dose/exposure. This study characterized astegolimab pharmacokinetics in patients with asthma and showed typical pharmacokinetic behavior as a monoclonal antibody-based drug. The exposure-response analyses suggested the highest dose tested in the Zenyatta study (490 mg every 4 weeks) performed close to the maximum effect, and no additional response may be expected above it.
Keywords: IL-33; ST2; astegolimab; asthma; exposure-response; population pharmacokinetics.
© 2021 Genentech Inc. The Journal of Clinical Pharmacology published by Wiley Periodicals LLC on behalf of American College of Clinical Pharmacology.
Conflict of interest statement
N.K. is a current employee of Chugai Pharmaceutical Co., Ltd. and was working at Genentech Inc. at the time of this study. M.D. was an employee of Genentech at the time of the study and owned stock in Roche Holding Ltd. (current affiliation: Roche Products Australia, Millers Point, NSW Australia). R.J.S. was an employee of Pharmetheus at the time of the study (current affiliation: the Swedish Medical Products Agency). J.R. is a current employee of Pharmetheus. L.E.F. is a professor at Uppsala University and a paid consultant to Pharmetheus. J.R. and L.E.F. own stocks in Pharmetheus. S.V., D.C., T.S., G.S., and J.J. are current Genentech employees and Roche shareholders. W.S.P. was an employee of Genentech at the time of the study and owned stock in Roche Holding Ltd. (current affiliation: Clinical Pharmacology and DMPK, Ultragenyx Pharmaceutical, Novato, USA). A.Q. was an employee of Genentech at the time of the study and owned stock in Roche Holding Ltd. (current affiliation: Clinical Pharmacology and Quantitative Pharmacology, AstraZeneca, Gothenburg, Sweden).
Figures

References
-
- Global strategy for asthma management and prevention, 2020 . Global Initiative for Asthma. www.ginasthma.org. Accessed November 19, 2021.
-
- Peters SP, Ferguson G, Deniz Y, Reisner C. Uncontrolled asthma: a review of the prevalence, disease burden and options for treatment. Respir Med. 2006;100:1139‐1151. - PubMed
-
- Zeiger RS, Schatz M, Li Q, et al. High blood eosinophil count is a risk factor for future asthma exacerbations in adult persistent asthma. J Allergy Clin Immunol Pract. 2014;2:741‐50. - PubMed
-
- Hermosa JL, Sánchez CB, Rubio MC, Mínguez MM, Walther JL. Factors associated with the control of severe asthma. J Asthma. 2010;47:124‐130. - PubMed

