Kinetics and persistence of anti-SARS-CoV-2 neutralisation and antibodies after BNT162b2 vaccination in a Swiss cohort
- PMID: 34965032
- PMCID: PMC8926495
- DOI: 10.1002/iid3.583
Kinetics and persistence of anti-SARS-CoV-2 neutralisation and antibodies after BNT162b2 vaccination in a Swiss cohort
Abstract
Introduction: Since the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), substantial effort has been made to gain knowledge about the immunity elicited by infection or vaccination.
Methods: We studied the kinetics of antibodies and virus neutralisation induced by vaccination with BNT162b2 in a Swiss cohort of SARS-CoV-2 naïve (n = 40) and convalescent (n = 9) persons. Blood sera were analysed in a live virus neutralisation assay and specific IgG and IgA levels were measured by enzyme-linked immunoassay and analysed by descriptive statistics.
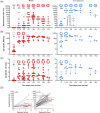
Results: Virus neutralisation was detected in all individuals 2-4 weeks after the second vaccine. Both neutralisation and antibodies remained positive for >4 months. Neutralisation and antibodies showed positive correlation, but immunoglobulin G (IgG) and immunoglobulin A (IgA) seroconversion took place 2-4 weeks faster than neutralisation. Spike-protein specific IgG levels rose significantly faster and were more stable over time than virus neutralisation titres or IgA responses. For naïve but not convalescent persons, a clear boosting effect was observed. Convalescent individuals showed faster, more robust and longer-lasting immune responses after vaccination compared to noninfected persons. No threshold could be determined for spike protein-specific IgG or IgA that would confer protection in the neutralisation assay, implicating the need for a better correlate of protection then antibody titres alone.
Conclusions: This study clearly shows the complex translation of antibody data and virus neutralisation, while supporting the evidence of a single dose being sufficient for effective antibody response in convalescent individuals.
Trial registration: ClinicalTrials.gov NCT04979871.
Keywords: BNT162b2; COVID-19; SARS-CoV-2; antibody response; mRNA vaccine; neutralisation assay.
© 2022 The Authors. Immunity, Inflammation and Disease published by John Wiley & Sons Ltd.
Conflict of interest statement
Senta M. Walton is employee of Saiba Biotech. Thomas M. Kündig is a medical advisor to Saiba Biotech, Pål Johansen has received financial support from PCI Biotech and Allergy Therapeutics. The other authors have no conflict of interests.
Figures



References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

