Cu-Catalyzed Site-Selective Benzylic Chlorination Enabling Net C-H Coupling with Oxidatively Sensitive Nucleophiles
- PMID: 34965136
- PMCID: PMC8830506
- DOI: 10.1021/acs.orglett.1c04038
Cu-Catalyzed Site-Selective Benzylic Chlorination Enabling Net C-H Coupling with Oxidatively Sensitive Nucleophiles
Abstract
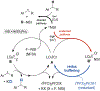
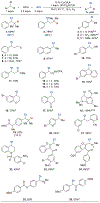
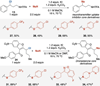
Site-selective chlorination of benzylic C-H bonds is achieved using a CuICl/bis(oxazoline) catalyst with N-fluorobenzenesulfonimide as the oxidant and KCl as a chloride source. This method exhibits higher benzylic selectivity, relative to established chlorination protocols, and is compatible with diverse alkyl arenes. Sequential benzylic C-H chlorination/nucleophilic substitution affords C-O, C-S, and C-N coupling products with oxidatively sensitive coupling partners.
Figures


References
-
- Schaumann E Science of Synthesis: Houben-Weyl Methods of Molecular Transformations Vol. 35: Chlorine, Bromine, and Iodine; Georg Thieme Verlag, 2014.
-
- Iwamoto H; Endo K; Ozawa Y; Watanabe Y; Kubota K; Imamoto T; Ito H Copper(I)-Catalyzed Enantioconvergent Borylation of Racemic Benzyl Chlorides Enabled by Quadrant-by-Quadrant Structure Modification of Chiral Bisphosphine Ligands. Angew. Chem., Int. Ed 2019, 55, 11112–11117. - PubMed
-
- Cybularczyk-Cecotka M; Szczepanik J; Giedyk M Photocatalytic Strategies for the Activation of Organic Chlorides. Nat Catal 2020, 3, 872–886.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

