Autophagy mediates ER stress and inflammation in Helicobacter pylori-related gastric cancer
- PMID: 34965181
- PMCID: PMC8726742
- DOI: 10.1080/19490976.2021.2015238
Autophagy mediates ER stress and inflammation in Helicobacter pylori-related gastric cancer
Abstract
Autophagy is a cellular degradation mechanism, which is triggered by the bacterium Helicobacter pylori. A single nucleotide polymorphism (SNP) in the autophagy gene ATG16L1 (rs2241880, G-allele) has been shown to dysregulate autophagy and increase intestinal endoplasmic reticulum (ER) stress. Here, we investigate the role of this SNP in H.pylori-mediated gastric carcinogenesis and its molecular pathways. ATG16L1 rs2241880 was genotyped in subjects from different ethnic cohorts (Dutch and Australian) presenting with gastric (pre)malignant lesions of various severity. Expression of GRP78 (a marker for ER stress) was assessed in gastric tissues. The effect of ATG16L1 rs2241880 on H.pylori-mediated ER stress and pro-inflammatory cytokine induction was investigated in organoids and CRISPR/Cas9 modified cell lines. Development of gastric cancer was associated with the ATG16L1 rs2241880 G-allele. Intestinal metaplastic cells in gastric tissue of patients showed increased levels of ER-stress. In vitro models showed that H.pylori increases autophagy while reducing ER stress, which appeared partly mediated by the ATG16L1 rs2241880 genotype. H.pylori-induced IL-8 production was increased while TNF-α production was decreased, in cells homozygous for the G-allele. The ATG16L1 rs2241880 G-allele is associated with progression of gastric premalignant lesions and cancer. Modulation of H.pylori-induced ER stress pathways and pro-inflammatory mediators by ATG16L1 rs2441880 may underlie this increased risk.
Keywords: ATG16L1; ER stress; Helicobacter pylori; Intestinal metaplasia; atrophic gastritis; autophagy; gastric cancer; inflammation.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


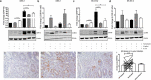
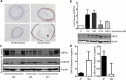
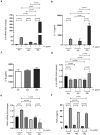

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
