The involvement of TGF-β1 /FAK/α-SMA pathway in the antifibrotic impact of rice bran oil on thioacetamide-induced liver fibrosis in rats
- PMID: 34965258
- PMCID: PMC8716044
- DOI: 10.1371/journal.pone.0260130
The involvement of TGF-β1 /FAK/α-SMA pathway in the antifibrotic impact of rice bran oil on thioacetamide-induced liver fibrosis in rats
Abstract
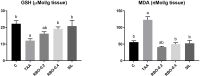
The objective of the current study is to investigate the effect of rice bran oil (RBO) on hepatic fibrosis as a characteristic response to persistent liver injuries. Rats were randomly allocated into five groups: the negative control group, thioacetamide (TAA) group (thioacetamide 100 mg/kg thrice weekly for two successive weeks, ip), RBO 0.2 and 0.4 groups (RBO 0.2mL and 0.4 mL/rat/day, po) and standard group (silymarin 100 mg/kg/day, po) for two weeks after TAA injection. Blood and liver tissue samples were collected for biochemical, molecular, and histological analyses. Liver functions, oxidative stress, inflammation, liver fibrosis markers were assessed. The obtained results showed that RBO reduced TAA-induced liver fibrosis and suppressed the extracellular matrix formation. Compared to the positive control group, RBO dramatically reduced total bilirubin, AST, and ALT blood levels. Furthermore, RBO reduced MDA and increased GSH contents in the liver. Simultaneously RBO downregulated the NF-κβ signaling pathway, which in turn inhibited the expression of some inflammatory mediators, including Cox-2, IL-1β, and TNF-α. RBO attenuated liver fibrosis by suppressing the biological effects of TGF-β1, α-SMA, collagen I, hydroxyproline, CTGF, and focal adhesion kinase (FAK). RBO reduced liver fibrosis by inhibiting hepatic stellate cell activation and modulating the interplay among the TGF-β1 and FAK signal transduction. The greater dosage of 0.4 mL/kg has a more substantial impact. Hence, this investigation presents RBO as a promising antifibrotic agent in the TAA model through inhibition of TGF-β1 /FAK/α-SMA.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


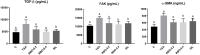





References
-
- Hessin A, Hegazy RR, Hassan AA, Yassin NZ, Kenawy SAB. Resveratrol prevents liver fibrosis via two possible pathways: Modulation of alpha fetoprotein transcriptional levels and normalization of protein kinase C responses. Indian J Pharmacol. 2017;49: 282–289. doi: 10.4103/ijp.IJP_299_16 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

