Pre-existing humoral immunity to human common cold coronaviruses negatively impacts the protective SARS-CoV-2 antibody response
- PMID: 34965382
- PMCID: PMC8648673
- DOI: 10.1016/j.chom.2021.12.005
Pre-existing humoral immunity to human common cold coronaviruses negatively impacts the protective SARS-CoV-2 antibody response
Abstract
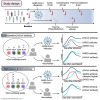
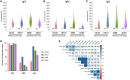
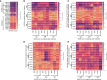
SARS-CoV-2 infection causes diverse outcomes ranging from asymptomatic infection to respiratory distress and death. A major unresolved question is whether prior immunity to endemic, human common cold coronaviruses (hCCCoVs) impacts susceptibility to SARS-CoV-2 infection or immunity following infection and vaccination. Therefore, we analyzed samples from the same individuals before and after SARS-CoV-2 infection or vaccination. We found hCCCoV antibody levels increase after SARS-CoV-2 exposure, demonstrating cross-reactivity. However, a case-control study indicates that baseline hCCCoV antibody levels are not associated with protection against SARS-CoV-2 infection. Rather, higher magnitudes of pre-existing betacoronavirus antibodies correlate with more SARS-CoV-2 antibodies following infection, an indicator of greater disease severity. Additionally, immunization with hCCCoV spike proteins before SARS-CoV-2 immunization impedes the generation of SARS-CoV-2-neutralizing antibodies in mice. Together, these data suggest that pre-existing hCCCoV antibodies hinder SARS-CoV-2 antibody-based immunity following infection and provide insight on how pre-existing coronavirus immunity impacts SARS-CoV-2 infection, which is critical considering emerging variants.
Keywords: 229E; COVID-19; HKU1; NL63; OC43; SARS-CoV-2; antibody; pre-existing immunity.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests P.G.T. has consulted for Illumina and 10X and serves on the advisory board of Immunoscape and Cyotagents. P.G.T. and J.C.C. filed patents related to treatment of severe respiratory infections, including SARS-CoV-2 (not based on research in this paper).
Figures





References
-
- Aguilar-Bretones M., Westerhuis B.M., Raadsen M.P., de Bruin E., Chandler F.D., Okba N.M., Haagmans B.L., Langerak T., Endeman H., van den Akker J.P., et al. Seasonal coronavirus-specific B cells with limited SARS-CoV-2 cross-reactivity dominate the IgG response in severe COVID-19. J. Clin. Invest. 2021;131:e150613. - PMC - PubMed
-
- Anderson E.M., Goodwin E.C., Verma A., Arevalo C.P., Bolton M.J., Weirick M.E., Gouma S., McAllister C.M., Christensen S.R., Weaver J., et al. UPenn COVID Processing Unit Seasonal human coronavirus antibodies are boosted upon SARS-CoV-2 infection but not associated with protection. Cell. 2021;184:1858–1864.e10. - PMC - PubMed
-
- Arvin A.M., Fink K., Schmid M.A., Cathcart A., Spreafico R., Havenar-Daughton C., Lanzavecchia A., Corti D., Virgin H.W. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature. 2020;584:353–363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

