Comparing self-reported reactogenicity between adolescents and adults following the use of BNT162b2 (Pfizer-BioNTech) messenger RNA COVID-19 vaccine: a prospective cohort study
- PMID: 34965462
- PMCID: PMC8710238
- DOI: 10.1016/j.ijid.2021.12.354
Comparing self-reported reactogenicity between adolescents and adults following the use of BNT162b2 (Pfizer-BioNTech) messenger RNA COVID-19 vaccine: a prospective cohort study
Abstract
Objectives: Although clinical data have shown that the BNT162b2 vaccine, which is widely used in many countries, is safe and effective as a protection against the SARS-CoV-2 infection, extant research in adverse reactions using real-world data of various sociodemographic characteristics is scant.
Methods: We conducted a prospective cohort study to compare age differences in self-reported reactogenicity of BNT162b2 in Hong Kong. A total of 1,516 participants were intensively followed up for two weeks following both doses of BNT162b2 vaccination, during which their basic demographic, health conditions, and medication information were collected.
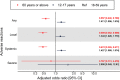
Results: Results from the generalized mixed model showed that compared with adults aged 18 to 59 years, older adults aged 60 years or above had a lower risk of adverse reactions and adolescents aged 12 to 17 years had a moderately higher risk.
Conclusions: Results of this study should be informative to parents considering BNT162b2 vaccination for their children in that moderately increased reactogenicity compared with adults is anticipated.
Keywords: COVID-19; epidemiology; pediatrics; pharmacovigilance; vaccine safety.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
Similar articles
-
Safety of COVID-19 Pfizer-BioNtech (BNT162b2) mRNA vaccination in adolescents aged 12-17 years: A systematic review and meta-analysis.Hum Vaccin Immunother. 2022 Nov 30;18(6):2144039. doi: 10.1080/21645515.2022.2144039. Epub 2022 Nov 11. Hum Vaccin Immunother. 2022. PMID: 36367429 Free PMC article.
-
Reactogenicity of the BNT162b2 mRNA vaccine (Pfizer-BioNTech) against COVID-19 in workers of a tertiary hospital.Farm Hosp. 2022 Mar 4;46(3):152-156. Farm Hosp. 2022. PMID: 36183208 English.
-
COVID-19 vaccine waning and effectiveness and side-effects of boosters: a prospective community study from the ZOE COVID Study.Lancet Infect Dis. 2022 Jul;22(7):1002-1010. doi: 10.1016/S1473-3099(22)00146-3. Epub 2022 Apr 8. Lancet Infect Dis. 2022. PMID: 35405090 Free PMC article.
-
Effectiveness of 2-Dose BNT162b2 (Pfizer BioNTech) mRNA Vaccine in Preventing SARS-CoV-2 Infection Among Children Aged 5-11 Years and Adolescents Aged 12-15 Years - PROTECT Cohort, July 2021-February 2022.MMWR Morb Mortal Wkly Rep. 2022 Mar 18;71(11):422-428. doi: 10.15585/mmwr.mm7111e1. MMWR Morb Mortal Wkly Rep. 2022. PMID: 35298453 Free PMC article.
-
Safety and reactogenicity of the BNT162b2 COVID-19 vaccine: Development, post-marketing surveillance, and real-world data.Hum Vaccin Immunother. 2024 Dec 31;20(1):2315659. doi: 10.1080/21645515.2024.2315659. Epub 2024 Feb 26. Hum Vaccin Immunother. 2024. PMID: 38407186 Free PMC article. Review.
Cited by
-
Autoimmune conditions following mRNA (BNT162b2) and inactivated (CoronaVac) COVID-19 vaccination: A descriptive cohort study among 1.1 million vaccinated people in Hong Kong.J Autoimmun. 2022 Jun;130:102830. doi: 10.1016/j.jaut.2022.102830. Epub 2022 Apr 14. J Autoimmun. 2022. PMID: 35461018 Free PMC article.
-
Immunogenicity, effectiveness, and safety of COVID-19 vaccines among children and adolescents aged 2-18 years: an updated systematic review and meta-analysis.World J Pediatr. 2023 Nov;19(11):1041-1054. doi: 10.1007/s12519-022-00680-9. Epub 2023 Feb 1. World J Pediatr. 2023. PMID: 36723827 Free PMC article.
-
Menstrual disturbances in 12- to 15-year-old girls after one dose of COVID-19 Comirnaty vaccine: Population-based cohort study in Norway.Vaccine. 2023 Jan 9;41(2):614-620. doi: 10.1016/j.vaccine.2022.11.068. Epub 2022 Dec 2. Vaccine. 2023. PMID: 36517325 Free PMC article. No abstract available.
-
Safety of COVID-19 Pfizer-BioNtech (BNT162b2) mRNA vaccination in adolescents aged 12-17 years: A systematic review and meta-analysis.Hum Vaccin Immunother. 2022 Nov 30;18(6):2144039. doi: 10.1080/21645515.2022.2144039. Epub 2022 Nov 11. Hum Vaccin Immunother. 2022. PMID: 36367429 Free PMC article.
-
COVID-19 vaccination and carditis in children and adolescents: a systematic review and meta-analysis.Clin Res Cardiol. 2022 Oct;111(10):1161-1173. doi: 10.1007/s00392-022-02070-7. Epub 2022 Jul 30. Clin Res Cardiol. 2022. PMID: 35906423 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous