The role of remote flavin adenine dinucleotide pieces in the oxidative decarboxylation catalyzed by salicylate hydroxylase
- PMID: 34965488
- PMCID: PMC8824312
- DOI: 10.1016/j.bioorg.2021.105561
The role of remote flavin adenine dinucleotide pieces in the oxidative decarboxylation catalyzed by salicylate hydroxylase
Abstract
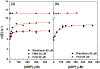
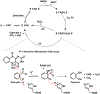
Salicylate hydroxylase (NahG) has a single redox site in which FAD is reduced by NADH, the O2 is activated by the reduced flavin, and salicylate undergoes an oxidative decarboxylation by a C(4a)-hydroperoxyflavin intermediate to give catechol. We report experimental results that show the contribution of individual pieces of the FAD cofactor to the observed enzymatic activity for turnover of the whole cofactor. A comparison of the kinetic parameters and products for the NahG-catalyzed reactions of FMN and riboflavin cofactor fragments reveal that the adenosine monophosphate (AMP) and ribitol phosphate pieces of FAD act to anchor the flavin to the enzyme and to direct the partitioning of the C(4a)-hydroperoxyflavin reaction intermediate towards hydroxylation of salicylate. The addition of AMP or ribitol phosphate pieces to solutions of the truncated flavins results in a partial restoration of the enzymatic activity lost upon truncation of FAD, and the pieces direct the reaction of the C(4a)-hydroperoxyflavin intermediate towards hydroxylation of salicylate.
Keywords: Activation; Biocatalysis; Flavoenzyme; One-component flavoprotein monooxygenase; Oxidoreductases.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
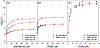

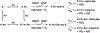
References
-
- Macheroux P, Kappes B, Ealick SE, Flavogenomics – a genomic and structural view of flavin-dependent proteins, FEBS J 278(15) (2011) 2625–2634. - PubMed
-
- Massey V, Activation of molecular oxygen by flavins and flavoproteins, J. Biol. Chem 269(36) (1994) 22459–62. - PubMed
-
- Romero E, Gómez Castellanos JR, Gadda G, Fraaije MW, Mattevi A, Same substrate, many reactions: Oxygen activation in flavoenzymes, Chem. Rev 118(4) (2018) 1742–1769. - PubMed
-
- Westphal AH, Tischler D, van Berkel WJH, Natural diversity of FAD-dependent 4-hydroxybenzoate hydroxylases, Arch. Biochem. Biophys 702 (2021). - PubMed
-
- Paul CE, Eggerichs D, Westphal AH, Tischler D, van Berkel WJH, Flavoprotein monooxygenases: Versatile biocatalysts, Biotechnol. Adv (2021) 107712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

