Development of a recombinant nucleocapsid protein-based ELISA for the detection of IgM and IgG antibodies to SARS-CoV-2
- PMID: 34965611
- PMCID: PMC9011413
- DOI: 10.1002/bab.2308
Development of a recombinant nucleocapsid protein-based ELISA for the detection of IgM and IgG antibodies to SARS-CoV-2
Abstract
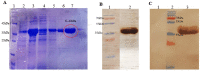
Coronavirus 2019 (COVID-19) is a global concern for public health. Thus, early and accurate diagnosis is a critical step in management of this infectious disease. Currently, RT-PCR is routine diagnosis test for COVID-19, but it has some limitations and false negative results. enzyme-linked immunosorbent assay (ELISA) against SARS-CoV-2 antigens seems to be an appropriate approach for serodiagnosis of COVID-19. In the current study, an ELISA system, using a recombinant nucleocapsid (N) protein, was developed for the detection of IgM and IgG antibodies to SARS-CoV-2. The related protein was expressed, purified, and used in an ELISA system. Sera samples (67) for COVID-19 patients, as well as sera samples from healthy volunteers (112), along with sera samples from non-COVID-19 patients were examined by the ELISA system. The expression and purity of the recombinant N protein were approved by SDS-PAGE and Western blotting. The sensitivity of ELISA system was 91.04 and 92.53% for the detection of IgG and IgM antibodies, respectively. Moreover, the specificity of the developed ELISA system for IgG and IgM were 98.21 and 97.32%, respectively. Our developed ELISA system showed satisfactory sensitivity and specificity for the detection of antiSARS-CoV-2 IgM and IgG antibodies and could be used as a complementary approach for proper diagnosis of COVID-19.
Keywords: COVID-19; ELISA; SARS-CoV-2; antibodies; nucleocapsid; recombinant.
© 2021 International Union of Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

