No evidence for motor-recovery-related cortical connectivity changes after stroke using resting-state fMRI
- PMID: 34965743
- PMCID: PMC8896990
- DOI: 10.1152/jn.00148.2021
No evidence for motor-recovery-related cortical connectivity changes after stroke using resting-state fMRI
Abstract
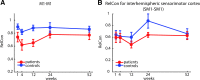
It has been proposed that a form of cortical reorganization (changes in functional connectivity between brain areas) can be assessed with resting-state (rs) functional MRI (fMRI). Here, we report a longitudinal data set collected from 19 patients with subcortical stroke and 11 controls. Patients were imaged up to five times over 1 year. We found no evidence, using rs-fMRI, for longitudinal poststroke cortical connectivity changes despite substantial behavioral recovery. These results could be construed as questioning the value of resting-state imaging. Here, we argue instead that they are consistent with other emerging reasons to challenge the idea of motor-recovery-related cortical reorganization poststroke when conceived of as changes in connectivity between cortical areas.NEW & NOTEWORTHY We investigated longitudinal changes in functional connectivity after stroke. Despite substantial motor recovery, we found no differences in functional connectivity patterns between patients and controls, nor any changes over time. Assuming that rs-fMRI is an adequate method to capture connectivity changes between cortical regions after brain injury, these results provide reason to doubt that changes in cortico-cortical connectivity are the relevant mechanism for promoting motor recovery.
Keywords: cortical reorganization; functional connectivity; motor recovery; resting-state imaging; stroke.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures






References
-
- Krakauer JW, Carmichael ST. Broken Movement. Cambridge, MA: MIT Press, 2017.
-
- Overman JJ, Clarkson AN, Wanner IB, Overman WT, Eckstein I, Maguire JL, Dinov ID, Toga AW, Carmichael ST. A role for ephrin-A5 in axonal sprouting, recovery, and activity-dependent plasticity after stroke. Proc Natl Acad Sci USA 109: E2230–E2239, 2012. doi:10.1073/pnas.1204386109. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

