Hereditary angioedema due to C1 inhibitor deficiency: real-world experience from the Icatibant Outcome Survey in Spain
- PMID: 34965883
- PMCID: PMC8715569
- DOI: 10.1186/s13223-021-00641-3
Hereditary angioedema due to C1 inhibitor deficiency: real-world experience from the Icatibant Outcome Survey in Spain
Abstract
Background: The Icatibant Outcome Survey (IOS) is an international registry monitoring the use of icatibant, a bradykinin B2 receptor antagonist indicated for the acute treatment of hereditary angioedema (HAE) attacks. Our goal was to assess disease characteristics and icatibant treatment outcomes in patients with HAE due to C1 inhibitor deficiency (HAE type 1 or 2 (HAE-1/2)) from Spain relative to other countries participating in IOS.
Methods: Descriptive retrospective analyses of data are reported from 10 centers in Spain vs 51 centers in 12 other participating countries (July 2009 to January 2019).
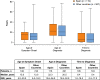
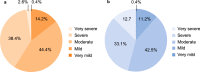
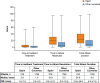
Results: No meaningful differences were identified between patients in Spain (n = 119) and patients across other countries (n = 907) regarding median age at symptom onset (15.0 vs 12.0 years) or diagnosis (22.3 vs 20.5 years). Overall HAE attack rates (total attacks/total years of follow-up) were 2.66 in Spain and 1.46 across other countries. Patients in Spain reported fewer severe/very severe HAE attacks before treatment (41.0% vs 45.9%; P < 0.0001) and, for icatibant-treated attacks, longer median time to treatment (2.9 vs 1.0 h), time to attack resolution (18.0 vs 5.5 h), and total attack duration (24.6 vs 8.0 h). Use of androgens for long-term prophylaxis was higher in Spain (51.2% vs 26.7%).
Conclusion: Patients with HAE-1/2 in Spain reported fewer severe/very severe attacks, administered icatibant later, and had longer-lasting attacks than did patients across other countries in IOS. These differences may indicate varying disease management practices (e.g., delayed icatibant treatment) and reporting. Efforts to raise awareness on the benefits of early on-demand treatment may be warranted.
Trial registration: NCT01034969.
Keywords: Bradykinin; Bradykinin B2 receptor antagonists; Hereditary angioedema; Icatibant; On-demand treatment; Registries; Spain.
© 2021. The Author(s).
Conflict of interest statement
MG has received speaker/consulting fees from CSL Behring, Novartis, and Takeda; received funding for travel/meeting attendance from CSL Behring and Takeda; and participated in clinical trials/registries for BioCryst, CSL Behring, Pharming, and Takeda. AS-C has received speaker/consulting fees from CSL Behring, Novartis, Sanofi, and Takeda; received funding for travel/meeting attendance from CSL Behring and Takeda; and participated in clinical trials/registries for BioCryst, CSL Behring, Pharming, and Takeda. MLB has received speaker/consultancy fees from CSL Behring, Novartis, and Takeda; has received funding to attend conferences/educational events from CSL Behring, LETI, Novartis, and Takeda; has participated as an investigator in clinical trials/registries for CSL Behring, BioCryst, Novartis, and Takeda; and is a researcher in the IiSGM research program. RC has received funding for travel/meeting attendance from CSL Behring, Novartis, and Takeda and participated in clinical trials/registries for BioCryst, CSL Behring, Pharming, and Takeda. MDH has received consulting/speaker fees and funding for meeting attendance from CSL Behring, Menarini, Novartis, and Takeda and participated in clinical trials/registries for ALK, Allergy Therapeutics, Bial, CSL Behring, GlaxoSmithKline, HAL Allergy Group, Stallergenes, Takeda, and Teva. EI has received speaker fees and funding for travel/meeting attendance from Takeda and has participated in clinical trials/registries for ALK, Bial, CSL Behring, GlaxoSmithKline, HAL Allergy Group, Novartis, Stallergenes, and Takeda. CHdL has received speaker fees and funding for travel/meeting attendance from Novartis and Takeda and participated in clinical trials/registries for Takeda. RL has received speaker/consulting fees and funding for travel/meeting attendance from CSL Behring and Takeda and participated in clinical trials/registries for BioCryst. TL has received speaker fees from ALK-Abelló, Meda, Novartis, and Takeda; received consulting fees from Takeda; received funding for travel/meeting attendance from ALK-Abelló, Dyater, LETI, Menarini, and Novartis; and participated in clinical trials/registries for Novartis and Takeda. LM has received speaker fees from Chiesi, Meda, Novartis, and Takeda; consulting fees from Takeda; and funding for travel/meeting attendance from Menarini, Novartis, and Takeda. BSdSP has received speaker fees and funding for travel/meeting attendance from Novartis and Takeda. JB and IA are full-time employees of Takeda and hold stock/stock options in Takeda. TC is a member of advisory boards for BioCryst, CSL Behring, Novartis, Octapharma, Pharming, and Takeda; is a member of speaker bureaus for CSL Behring, Novartis, and Takeda; has received grants or honoraria from BioCryst, CSL Behring, Novartis, and Takeda; has received funding to attend conferences/educational events from CSL Behring, Novartis, and Takeda; is/has been a clinical trial/registry investigator for BioCryst, CSL Behring, Novartis, Pharming, and Takeda; and is a researcher from the IdiPAZ program for promoting research activities.
Figures
References
-
- Zanichelli A, Longhurst HJ, Maurer M, Bouillet L, Aberer W, Fabien V, et al; IOS Study Group. Misdiagnosis trends in patients with hereditary angioedema from the real-world clinical setting. Ann Allergy Asthma Immunol. 2016;117(4):394–8. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

