Plasma Protein Biomarkers in Advanced or Metastatic Colorectal Cancer Patients Receiving Chemotherapy With Bevacizumab or Cetuximab: Results from CALGB 80405 (Alliance)
- PMID: 34965954
- PMCID: PMC9240111
- DOI: 10.1158/1078-0432.CCR-21-2389
Plasma Protein Biomarkers in Advanced or Metastatic Colorectal Cancer Patients Receiving Chemotherapy With Bevacizumab or Cetuximab: Results from CALGB 80405 (Alliance)
Abstract
Purpose: CALGB 80405 compared the combination of first-line chemotherapy with cetuximab or bevacizumab in the treatment of advanced or metastatic colorectal cancer (mCRC). Although similar clinical outcomes were observed in the cetuximab-chemotherapy group and the bevacizumab-chemotherapy group, biomarkers could identify patients deriving more benefit from either biologic agent.
Patients and methods: In this exploratory analysis, the Angiome, a panel of 24 soluble protein biomarkers were measured in baseline plasma samples in CALGB 80405. Prognostic biomarkers were determined using univariate Cox proportional hazards models. Predictive biomarkers were identified using multivariable Cox regression models including interaction between biomarker level and treatment.
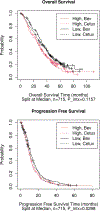
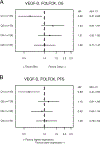
Results: In the total population, high plasma levels of Ang-2, CD73, HGF, ICAM-1, IL6, OPN, TIMP-1, TSP-2, VCAM-1, and VEGF-R3 were identified as prognostic of worse progression-free survival (PFS) and overall survival (OS). PlGF was identified as predictive of lack of PFS benefit from bevacizumab [bevacizumab HR, 1.51; 95% confidence interval (CI), 1.10-2.06; cetuximab HR, 0.94; 95% CI, 0.71-1.25; Pinteraction = 0.0298] in the combined FOLFIRI/FOLFOX regimens. High levels of VEGF-D were predictive of lack of PFS benefit from bevacizumab in patients receiving FOLFOX regimen only (FOLFOX/bevacizumab HR, 1.70; 95% CI, 1.19-2.42; FOLFOX/cetuximab HR, 0.92; 95% CI, 0.68-1.24; Pinteraction = 0.0097).
Conclusions: In this exploratory, hypothesis-generating analysis, the Angiome identified multiple prognostic biomarkers and two potential predictive biomarkers for patients with mCRC enrolled in CALGB 80405. PlGF and VEGF-D predicted lack of benefit from bevacizumab in a chemo-dependent manner. See related commentaries by Mishkin and Kohn, p. 2722 and George and Bertagnolli, p. 2725.
©2021 American Association for Cancer Research.
Figures


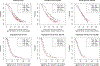
Comment in
-
Linking Genotype to Phenotype: Bench to Bedside.Clin Cancer Res. 2022 Jul 1;28(13):2725-2727. doi: 10.1158/1078-0432.CCR-22-0027. Clin Cancer Res. 2022. PMID: 35467726
-
Biomarker Development: Bedside to Bench.Clin Cancer Res. 2022 Jul 1;28(13):2722-2724. doi: 10.1158/1078-0432.CCR-22-0750. Clin Cancer Res. 2022. PMID: 35481871
References
-
- Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 2000;355(9209):1041–7 doi 10.1016/s0140-6736(00)02034-1. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UG1 CA233290/CA/NCI NIH HHS/United States
- UG1 CA189862/CA/NCI NIH HHS/United States
- UG1 CA180830/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- UG1 CA233373/CA/NCI NIH HHS/United States
- UG1 CA233180/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- U24 CA196171/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- UG1 CA233253/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- U10 CA180819/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- UG1 CA233337/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

