Distinct Factors Drive the Spatiotemporal Progression of Tau Pathology in Older Adults
- PMID: 34965972
- PMCID: PMC8883857
- DOI: 10.1523/JNEUROSCI.1601-21.2021
Distinct Factors Drive the Spatiotemporal Progression of Tau Pathology in Older Adults
Abstract
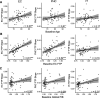
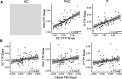
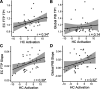
Mechanisms underlying the initial accumulation of tau pathology across the human brain are largely unknown. We examined whether baseline factors including age, amyloid-β (Aβ), and neural activity predicted longitudinal tau accumulation in temporal lobe regions that reflect distinct stages of tau pathogenesis. Seventy cognitively normal human older adults (77 ± 6 years, 59% female) received two or more 18F-flortaucipir (FTP) and 11C-Pittsburgh Compound B (PiB) PET scans (mean follow-up, 2.5 ± 1.1 years) to quantify tau and (Aβ). Linear mixed-effects models were used to calculate the slopes of FTP change in entorhinal cortex (EC), parahippocampal cortex (PHC), and inferior temporal gyrus (IT), and slopes of global PiB change. Thirty-seven participants underwent functional MRI to measure baseline activation. Older age predicted EC tau accumulation, and baseline EC tau levels predicted subsequent tau accumulation in EC and PHC. In IT, however, baseline EC tau interacted with Aβ to predict IT tau accumulation. Higher baseline local activation predicted tau accumulation within EC and PHC, and higher baseline hippocampal activation predicted EC tau accumulation. Our findings indicate that factors predicting tau accumulation vary as tau progresses through the temporal lobe. Older age is associated with initial tau accumulation in EC, while baseline EC tau and neural activity drive tau accumulation within medial temporal lobe. Aβ subsequently facilitates tau spread from medial to lateral temporal lobe. Our findings elucidate potential drivers of tau accumulation and spread in aging, which are critical for understanding Alzheimer's disease pathogenesis.SIGNIFICANCE STATEMENT To further understand the mechanisms leading to tau pathogenesis and spread, we tested whether baseline factors such as age, amyloid-β pathology, and activation predicted longitudinal tau accumulation in cognitively normal older adults. We found that distinct mechanisms contribute to tau accumulation as tau progresses across the temporal lobe, with initial tau accumulation in entorhinal cortex driven by age and subsequent spread driven by neural activity and amyloid-β. We demonstrate that higher baseline activation predicts increased longitudinal tau accumulation, providing novel evidence that activation-dependent tau production may occur in the human brain. Our findings support major hypotheses generated from preclinical research, and have important translational implications, suggesting that the reduction of hyperactivation may help prevent the development of tau pathology.
Keywords: Alzheimer's disease; PET; aging; amyloid-β; fMRI; tau.
Copyright © 2022 the authors.
Figures