Reduced Osteogenic Differentiation Potential In Vivo in Acute Myeloid Leukaemia Patients Correlates with Decreased BMP4 Expression in Mesenchymal Stromal Cells
- PMID: 34966001
- PMCID: PMC9148835
- DOI: 10.15283/ijsc21138
Reduced Osteogenic Differentiation Potential In Vivo in Acute Myeloid Leukaemia Patients Correlates with Decreased BMP4 Expression in Mesenchymal Stromal Cells
Abstract
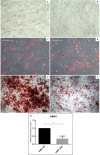
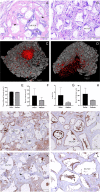
The osteogenic differentiation potential of mesenchymal stromal cells (hMSCs) is an essential process for the haematopoiesis and the maintenance of haematopoietic stem cells (HSCs). Therefore, the aim of this work was to evaluate this potential in hMSCs from AML patients (hMSCs-AML) and whether it is associated with BMP4 expression. The results showed that bone formation potential in vivo was reduced in hMSCs-AML compared to hMSCs from healthy donors (hMSCs-HD). Moreover, the fact that hMSCs-AML were not able to develop supportive haematopoietic cells or to differentiate into osteocytes suggests possible changes in the bone marrow microenvironment. Furthermore, the expression of BMP4 was decreased, indicating a lack of gene expression committed to the osteogenic lineage. Overall, these alterations could be associated with changes in the maintenance of HSCs, the leukaemic transformation process and the development of AML.
Keywords:
Acute myeloid leukaemia (AML);
Conflict of interest statement
The authors have no conflicting financial interest.
Figures
References
-
- Shlush LI, Mitchell A, Heisler L, Abelson S, Ng SWK, Trotman-Grant A, Medeiros JJF, Rao-Bhatia A, Jaciw-Zurakowsky I, Marke R, McLeod JL, Doedens M, Bader G, Voisin V, Xu C, McPherson JD, Hudson TJ, Wang JCY, Minden MD, Dick JE. Tracing the origins of relapse in acute myeloid leukaemia to stem cells. Nature. 2017;547:104–108. doi: 10.1038/nature22993. - DOI - PubMed
-
- Geyh S, Rodríguez-Paredes M, Jäger P, Khandanpour C, Cadeddu RP, Gutekunst J, Wilk CM, Fenk R, Zilkens C, Hermsen D, Germing U, Kobbe G, Lyko F, Haas R, Schroeder T. Functional inhibition of mesenchymal stromal cells in acute myeloid leukemia. Leukemia. 2016;30:683–691. doi: 10.1038/leu.2015.325. - DOI - PubMed
LinkOut - more resources
Full Text Sources

