BpOmpW Antigen Stimulates the Necessary Protective T-Cell Responses Against Melioidosis
- PMID: 34966388
- PMCID: PMC8710444
- DOI: 10.3389/fimmu.2021.767359
BpOmpW Antigen Stimulates the Necessary Protective T-Cell Responses Against Melioidosis
Abstract
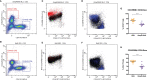
Melioidosis is a potentially fatal bacterial disease caused by Burkholderia pseudomallei and is estimated to cause 89,000 deaths per year in endemic areas of Southeast Asia and Northern Australia. People with diabetes mellitus are most at risk of melioidosis, with a 12-fold increased susceptibility for severe disease. Interferon gamma (IFN-γ) responses from CD4 and CD8 T cells, but also from natural killer (NK) and natural killer T (NKT) cells, are necessary to eliminate the pathogen. We previously reported that immunization with B. pseudomallei OmpW (BpOmpW antigen) protected mice from lethal B. pseudomallei challenge for up to 81 days. Elucidating the immune correlates of protection of the protective BpOmpW vaccine is an essential step prior to clinical trials. Thus, we immunized either non-insulin-resistant C57BL/6J mice or an insulin-resistant C57BL/6J mouse model of type 2 diabetes (T2D) with a single dose of BpOmpW. BpOmpW induced strong antibody responses, stimulated effector CD4+ and CD8+ T cells and CD4+ CD25+ Foxp3+ regulatory T cells, and produced higher IFN-γ responses in CD4+, CD8+, NK, and NKT cells in non-insulin-resistant mice. The T-cell responses of insulin-resistant mice to BpOmpW were comparable to those of non-insulin-resistant mice. In addition, as a precursor to its evaluation in human studies, humanized HLA-DR and HLA-DQ (human leukocyte antigen DR and DQ isotypes, respectively) transgenic mice elicited IFN-γ recall responses in an enzyme-linked immune absorbent spot (ELISpot)-based study. Moreover, human donor peripheral blood mononuclear cells (PBMCs) exposed to BpOmpW for 7 days showed T-cell proliferation. Finally, plasma from melioidosis survivors with diabetes recognized our BpOmpW vaccine antigen. Overall, the range of approaches used strongly indicated that BpOmpW elicits the necessary immune responses to combat melioidosis and bring this vaccine closer to clinical trials.
Keywords: Burkholderia pseudomallei; IFN-γ; T-cell responses; melioidosis; type 2 diabetes; vaccine.
Copyright © 2021 Tomás-Cortázar, Bossi, Quinn, Reynolds, Butler, Corcoran, Murchú, McMahon, Singh, Rongkard, Anguita, Blanco, Dunachie, Altmann, Boyton, Arnold, Giltaire and McClean.
Conflict of interest statement
Author MS was employed by company LIONEX Diagnostics and Therapeutics GmbH. Authors LB, JhA and SG were employed by Immunxperts company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

