To Predict Anti-Inflammatory and Immunomodulatory Targets of Guizhi Decoction in Treating Asthma Based on Network Pharmacology, Molecular Docking, and Experimental Validation
- PMID: 34966437
- PMCID: PMC8712140
- DOI: 10.1155/2021/9033842
To Predict Anti-Inflammatory and Immunomodulatory Targets of Guizhi Decoction in Treating Asthma Based on Network Pharmacology, Molecular Docking, and Experimental Validation
Abstract
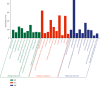
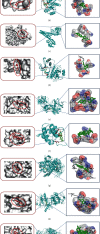
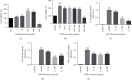
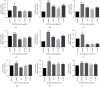
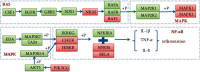
Asthma, characterized by the continuous inflammatory response caused by a variety of immune cells, is one of the most common chronic respiratory diseases worldwide. Relevant clinical trials proved that the traditional Chinese medicine formula Guizhi Decoction (GZD) had multitarget and multichannel functions, which might be an effective drug for asthma. However, the effective ingredients and mechanisms of GZD against asthma are still unclear. Therefore, network pharmacology, molecular docking, and cell experiments were performed to explore the antiasthma effects and potential mechanisms of GZD. First, we applied the TCMSP database and literature to obtain the bioactivated ingredients in GZD. SwissTargetPrediction, TCMSP, GeneCards, OMIM, PharmGkb, TTD, DrugBank, and STRING database were used to get core genes. In addition, the key pathways were analyzed by the DAVID database. Molecular docking was used to predict whether the important components could act on the core target proteins directly. Finally, qPCR was carried out to verify the network pharmacology results and the possible mechanisms of GZD in the treatment of asthma. We collected 134 active ingredients in GZD, 959 drug targets, and 3223 disease targets. 431 intersection genes were screened for subsequent analysis. Through GO and KEGG analyses, enriched pathways related to inflammation and immune regulation were presented. Through the qPCR method to verify the role of essential genes, we found that GZD had an excellent anti-inflammatory effect. Direct or indirect inhibition of MAPK and NF-κB pathways might be one of the crucial mechanisms of GZD against asthma. GZD might be a promising potential drug for the treatment of asthma. This article provided a reference for the clinical application of GZD.
Copyright © 2021 Rui Sun et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
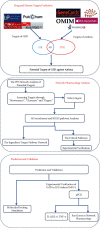

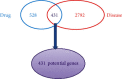

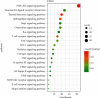


References
-
- Corren J. Asthma phenotypes and endotypes: an evolving paradigm for classification. Discovery Medicine . 2013;15(83):243–249. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

