LAR Receptor Tyrosine Phosphatase Family in Healthy and Diseased Brain
- PMID: 34966732
- PMCID: PMC8711739
- DOI: 10.3389/fcell.2021.659951
LAR Receptor Tyrosine Phosphatase Family in Healthy and Diseased Brain
Abstract
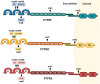
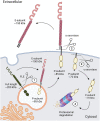
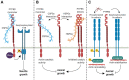
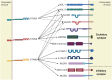
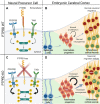
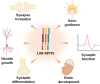
Protein phosphatases are major regulators of signal transduction and they are involved in key cellular mechanisms such as proliferation, differentiation, and cell survival. Here we focus on one class of protein phosphatases, the type IIA Receptor-type Protein Tyrosine Phosphatases (RPTPs), or LAR-RPTP subfamily. In the last decade, LAR-RPTPs have been demonstrated to have great importance in neurobiology, from neurodevelopment to brain disorders. In vertebrates, the LAR-RPTP subfamily is composed of three members: PTPRF (LAR), PTPRD (PTPδ) and PTPRS (PTPσ), and all participate in several brain functions. In this review we describe the structure and proteolytic processing of the LAR-RPTP subfamily, their alternative splicing and enzymatic regulation. Also, we review the role of the LAR-RPTP subfamily in neural function such as dendrite and axon growth and guidance, synapse formation and differentiation, their participation in synaptic activity, and in brain development, discussing controversial findings and commenting on the most recent studies in the field. Finally, we discuss the clinical outcomes of LAR-RPTP mutations, which are associated with several brain disorders.
Keywords: PTPdelta; PTPsigma; brain disorders; protein phosphatase; receptor protein tyrosine phosphatase (RPTP).
Copyright © 2021 Cornejo, Cortés, Findlay and Cancino.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

