Outcomes of SOT Recipients With COVID-19 in Different Eras of COVID-19 Therapeutics
- PMID: 34966840
- PMCID: PMC8710330
- DOI: 10.1097/TXD.0000000000001268
Outcomes of SOT Recipients With COVID-19 in Different Eras of COVID-19 Therapeutics
Abstract
Background: Few reports have focused on newer coronavirus disease 2019 (COVID-19) therapies (remdesivir, dexamethasone, and convalescent plasma) in solid organ transplant recipients; concerns had been raised regarding possible adverse impact on allograft function or secondary infections.
Methods: We studied 77 solid organ transplant inpatients with COVID-19 during 2 therapeutic eras (Era 1: March-May 2020, 21 patients; and Era 2: June-November 2020, 56 patients) and 52 solid organ transplant outpatients.
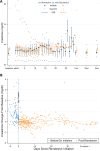
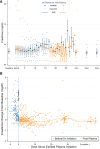
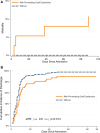
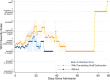
Results: In Era 1, no patients received remdesivir or dexamethasone, and 4 of 21 (19.4%) received convalescent plasma, whereas in Era 2, remdesivir (24/56, 42.9%), dexamethasone (24/56, 42.9%), and convalescent plasma (40/56, 71.4%) were commonly used. Mortality was low across both eras, 4 of 77 (5.6%), and rejection occurred in only 2 of 77 (2.8%) inpatients; infections were similar in hypoxemic patients with or without dexamethasone. Preexisting graft dysfunction was associated with greater need for hospitalization, higher severity score, and lower survival. Acute kidney injury was present in 37.3% of inpatients; renal function improved more rapidly in patients who received remdesivir and convalescent plasma. Post-COVID-19 renal and liver function were comparable between eras, out to 90 d.
Conclusions: Newer COVID-19 therapies did not appear to have a deleterious effect on allograft function, and infectious complications were comparable.
Copyright © 2021 The Author(s). Transplantation Direct. Published by Wolters Kluwer Health, Inc.
Figures
References
-
- Avery RK. COVID-19 therapeutics for solid organ transplant recipients; 6 months into the pandemic: where are we now? Transplantation. 2021;105:56–60. - PubMed
-
- Crespo M, Mazuecos A, Rodrigo E, et al. ; Spanish Society of Nephrology COVID-19 Group. Respiratory and gastrointestinal COVID-19 phenotypes in kidney transplant recipients. Transplantation. 2020;104:2225–2233. - PubMed

