The 2021 IXA Keith Reemtsma Lecture: Moving xenotransplantation to the clinic
- PMID: 34967057
- PMCID: PMC8995333
- DOI: 10.1111/xen.12723
The 2021 IXA Keith Reemtsma Lecture: Moving xenotransplantation to the clinic
Abstract
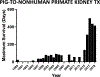
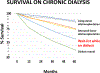
Keith Reemtsma was a pioneer in xenotransplantation, the Honorary Founding President of the International Xenotransplantation Association (in 1998), and a wonderful personality. It is a privilege to be invited to give this lecture in his memory. If he were alive today, he would be delighted to see the progress that has been made in pig organ transplantation into nonhuman primate recipients. This progress has largely resulted from two major advances: (i) the increasing availability of pigs with multiple genetic manipulations aimed at protecting the cells of the organ from the primate immune response and (ii) the introduction of novel immunosuppressive agents that block the CD40/CD154 costimulation pathway. There is strong evidence from numerous in vitro studies that the transplantation of a triple-knockout pig organ, particularly if expressing several human protective proteins, into a patient is likely to be significantly more successful than if that same organ is transplanted into a nonhuman primate recipient. With this fact in mind, and in view of the advances currently being made, the time has surely come when we need to consider moving from the laboratory to the clinic. However, there are still questions we need to definitively resolve: (i) What exact genetic modifications do we need in the organ-source pig? (ii) What exact immunosuppressive regimen will we choose? (iii) How will we monitor the immune response and diagnose and treat rejection? and (iv) How do we plan to prevent or treat potential infectious complications? Furthermore, when these matters have been resolved, which patients will be offered a pig organ in the first trial? We have suggested that patients who are very unlikely to survive until a suitable deceased human donor kidney becomes available are those who should be considered for the initial trials. Assessing public attitudes to xenotransplantation is also important before embarking on a clinical trial. I suggest that progress is much more likely to be made from a small clinical trial than if we persist in carrying out experiments in an animal model that no longer mimics the clinical situation.
Keywords: International Xenotransplantation Association; Keith Reemtsma; nonhuman primate; pig; xenotransplantation.
© 2021 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
Conflict of interest statement
The author is a consultant to eGenesis Bio, Cambridge, MA, USA, but the opinions he has expressed in this article are his own and do not necessarily represent the views of eGenesis Bio.
Figures






References
-
- Hardy MA. The ‘Reemtsma Era’: recollections of an acolyte. In Cooper DKC (ed) Recollections of Pioneers in Xenotransplantation. Nova, New York, 2018, pp1–20
-
- Lexer G, Cooper DKC, Rose AG, et al. Hyperacute rejection in a discordant (pig to baboon) cardiac xenograft model. J Heart Transplant 1986;5:411–418. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

