Weight loss and high-protein, high-fiber diet consumption impact blood metabolite profiles, body composition, voluntary physical activity, fecal microbiota, and fecal metabolites of adult dogs
- PMID: 34967874
- PMCID: PMC8846339
- DOI: 10.1093/jas/skab379
Weight loss and high-protein, high-fiber diet consumption impact blood metabolite profiles, body composition, voluntary physical activity, fecal microbiota, and fecal metabolites of adult dogs
Abstract
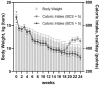
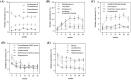
Canine obesity is associated with reduced lifespan and metabolic dysfunction, but can be managed by dietary intervention. This study aimed to determine the effects of restricted feeding of a high-protein, high-fiber (HPHF) diet and weight loss on body composition, physical activity, blood metabolites, and fecal microbiota and metabolites of overweight dogs. Twelve spayed female dogs (age: 5.5 ± 1.1 yr; body weight [BW]: 14.8 ± 2.0 kg, body condition score [BCS]: 7.9 ± 0.8) were fed a HPHF diet during a 4-wk baseline phase to maintain BW. After baseline (week 0), dogs were first fed 80% of baseline intake and then adjusted to target 1.5% weekly weight loss for 24 wk. Body composition using dual-energy x-ray absorptiometry and blood samples (weeks 0, 6, 12, 18, and 24), voluntary physical activity (weeks 0, 7, 15, and 23), and fresh fecal samples for microbiota and metabolite analysis (weeks 0, 4, 8, 12, 16, 20, and 24) were measured over time. Microbiota data were analyzed using QIIME 2. All data were analyzed statistically over time using SAS 9.4. After 24 wk, dogs lost 31.2% of initial BW and had 1.43 ± 0.73% weight loss per week. BCS decreased (P < 0.0001) by 2.7 units, fat mass decreased (P < 0.0001) by 3.1 kg, and fat percentage decreased (P < 0.0001) by 11.7% with weight loss. Many serum metabolites and hormones were altered, with triglycerides, leptin, insulin, C-reactive protein, and interleukin-6 decreasing (P < 0.05) with weight loss. Relative abundances of fecal Bifidobacterium, Coriobacteriaceae UCG-002, undefined Muribaculaceae, Allobaculum, Eubacterium, Lachnospira, Negativivibacillus, Ruminococcus gauvreauii group, uncultured Erysipelotrichaceae, and Parasutterella increased (P < 0.05), whereas Prevotellaceae Ga6A1 group, Catenibacterium, Erysipelatoclostridium, Fusobacterium, Holdemanella, Lachnoclostridium, Lactobacillus, Megamonas, Peptoclostridium, Ruminococcus gnavus group, and Streptococcus decreased (P < 0.01) with weight loss. Despite the number of significant changes, a state of dysbiosis was not observed in overweight dogs. Fecal ammonia and secondary bile acids decreased, whereas fecal valerate increased with weight loss. Several correlations between gut microbial taxa and biological parameters were observed. Our results suggest that restricted feeding of a HPHF diet and weight loss promotes fat mass loss, minimizes lean mass loss, reduces inflammatory marker and triglyceride concentrations, and modulates fecal microbiota phylogeny and activity in overweight dogs.
Keywords: caloric restriction; canine nutrition; dietary fiber; high-protein diet; weight loss.
Plain language summary
Canine obesity is associated with reduced lifespan and metabolic dysfunction, but dietary intervention may aid in its management. This study aimed to determine the effects of restricted feeding of a high-protein, high-fiber (HPHF) diet and weight loss on body composition, physical activity, blood metabolites, and fecal bacteria and metabolites of overweight dogs. Twelve overweight dogs were fed a HPHF diet during a 4-wk baseline to maintain body weight and then fed to lose weight for 24 wk. Body composition, blood samples, voluntary physical activity, and fresh fecal samples were measured over time. After 24 wk, dogs lost over 30% of their initial body weight and had 1.4% weight loss per week. As expected, serum triglycerides, leptin, insulin, C-reactive protein, and interleukin-6 decreased with weight loss. The relative abundances of 4 bacterial phyla and over 30 bacterial genera were altered with weight loss. Fecal ammonia and secondary bile acid concentrations decreased, whereas fecal valerate concentrations increased with weight loss. Several correlations between fecal bacteria and physiological parameters were identified. Our results suggest that a HPHF diet and weight loss promote fat mass loss, reduce inflammatory marker and triglyceride concentrations, and modulate fecal bacterial populations and activity in overweight dogs.
© The Author(s) 2021. Published by Oxford University Press on behalf of the American Society of Animal Science. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

Similar articles
-
Effects of weight loss and feeding specially formulated diets on the body composition, blood metabolite profiles, voluntary physical activity, and fecal metabolites and microbiota of obese dogs.J Anim Sci. 2023 Jan 3;101:skad073. doi: 10.1093/jas/skad073. J Anim Sci. 2023. PMID: 36879442 Free PMC article.
-
Effects of weight loss and feeding specially formulated diets on the body composition, blood metabolite profiles, voluntary physical activity, and fecal metabolites and microbiota of overweight cats.J Anim Sci. 2023 Jan 3;101:skad332. doi: 10.1093/jas/skad332. J Anim Sci. 2023. PMID: 37773637 Free PMC article.
-
Restricted feeding of weight control diets induces weight loss and affects body composition, voluntary physical activity, blood metabolites, hormones, and oxidative stress markers, and fecal metabolites and microbiota of obese cats.J Anim Sci. 2024 Jan 3;102:skae335. doi: 10.1093/jas/skae335. J Anim Sci. 2024. PMID: 39485233
-
Diet and the gut microbiota profiles in individuals at risk of chronic heart failure - A review on the Asian population.Asia Pac J Clin Nutr. 2025 Apr;34(2):141-152. doi: 10.6133/apjcn.202504_34(2).0001. Asia Pac J Clin Nutr. 2025. PMID: 40134053 Free PMC article. Review.
-
Effects of Animal and Vegetable Proteins on Gut Microbiota in Subjects with Overweight or Obesity.Nutrients. 2023 Jun 8;15(12):2675. doi: 10.3390/nu15122675. Nutrients. 2023. PMID: 37375578 Free PMC article. Review.
Cited by
-
Case Report: Fecal Microbiota Transplantation for the Treatment of Generalized Eczema Occurring After COVID-19 Vaccination.Clin Cosmet Investig Dermatol. 2024 Jan 26;17:229-235. doi: 10.2147/CCID.S443542. eCollection 2024. Clin Cosmet Investig Dermatol. 2024. PMID: 38292322 Free PMC article.
-
Effect of Different Fiber Sources as Additives to Wet Food for Beagle Dogs on Diet Acceptance, Digestibility, and Fecal Quality.Vet Sci. 2023 Jan 25;10(2):91. doi: 10.3390/vetsci10020091. Vet Sci. 2023. PMID: 36851395 Free PMC article.
-
Effects of feeding Candida utilis-fermented pea starch on overall, metabolic and intestinal health of dogs and cats.Front Vet Sci. 2025 Jun 9;12:1542484. doi: 10.3389/fvets.2025.1542484. eCollection 2025. Front Vet Sci. 2025. PMID: 40552080 Free PMC article.
-
Collaborative Metabolism: Gut Microbes Play a Key Role in Canine and Feline Bile Acid Metabolism.Vet Sci. 2024 Feb 18;11(2):94. doi: 10.3390/vetsci11020094. Vet Sci. 2024. PMID: 38393112 Free PMC article. Review.
-
A protein- and fiber-rich diet with astaxanthin alleviates high-fat diet-induced obesity in beagles.Front Nutr. 2022 Oct 24;9:1019615. doi: 10.3389/fnut.2022.1019615. eCollection 2022. Front Nutr. 2022. PMID: 36352906 Free PMC article.
References
-
- AAFCO . 2018. Official Publication. Oxford (IN): Association of American Feed Control Officials, Inc.
-
- American Animal Hospital Association (AAHA) . 2003. The Path to High-Quality Care: practical tips for improving compliance. Lakewood (CO): American Animal Hospital Association.
-
- American Association of Cereal Chemists (AACC) . 1983. Approved methods. 8th ed. St. Paul (MN): Amer. Assoc. Cereal Chem.
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

