The LGI1 protein: molecular structure, physiological functions and disruption-related seizures
- PMID: 34967933
- PMCID: PMC11072701
- DOI: 10.1007/s00018-021-04088-y
The LGI1 protein: molecular structure, physiological functions and disruption-related seizures
Abstract
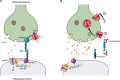
Leucine-rich, glioma inactivated 1 (LGI1) is a secreted glycoprotein, mainly expressed in the brain, and involved in central nervous system development and physiology. Mutations of LGI1 have been linked to autosomal dominant lateral temporal lobe epilepsy (ADLTE). Recently auto-antibodies against LGI1 have been described as the basis for an autoimmune encephalitis, associated with specific motor and limbic epileptic seizures. It is the second most common cause of autoimmune encephalitis. This review presents details on the molecular structure, expression and physiological functions of LGI1, and examines how their disruption underlies human pathologies. Knock-down of LGI1 in rodents reveals that this protein is necessary for normal brain development. In mature brains, LGI1 is associated with Kv1 channels and AMPA receptors, via domain-specific interaction with membrane anchoring proteins and contributes to regulation of the expression and function of these channels. Loss of function, due to mutations or autoantibodies, of this key protein in the control of neuronal activity is a common feature in the genesis of epileptic seizures in ADLTE and anti-LGI1 autoimmune encephalitis.
Keywords: AMPA receptors; Autoimmune encephalitis; Genetic epilepsy; Kv1; LGI1.
© 2021. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
V.N. reports personal fees from UCB, Liva Nova, and EISAI, outside the submitted work. The other authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

