Modulating Angiogenesis by Proteomimetics of Vascular Endothelial Growth Factor
- PMID: 34968032
- PMCID: PMC8886800
- DOI: 10.1021/jacs.1c09571
Modulating Angiogenesis by Proteomimetics of Vascular Endothelial Growth Factor
Abstract
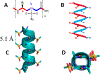
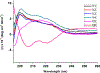
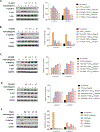
Angiogenesis, formation of new blood vessels from the existing vascular network, is a hallmark of cancer cells that leads to tumor vascular proliferation and metastasis. This process is mediated through the binding interaction of VEGF-A with VEGF receptors. However, the balance between pro-angiogenic and anti-angiogenic effect after ligand binding yet remains elusive and is therefore challenging to manipulate. To interrogate this interaction, herein we designed a few sulfono-γ-AA peptide based helical peptidomimetics that could effectively mimic a key binding interface on VEGF (helix-α1) for VEGFR recognition. Intriguingly, although both sulfono-γ-AA peptide sequences V2 and V3 bound to VEGF receptors tightly, in vitro angiogenesis assays demonstrated that V3 potently inhibited angiogenesis, whereas V2 activated angiogenesis effectively instead. Our findings suggested that this distinct modulation of angiogenesis might be due to the result of selective binding of V2 to VEGFR-1 and V3 to VEGFR-2, respectively. These molecules thus provide us a key to switch the angiogenic signaling, a biological process that balances the effects of pro-angiogenic and anti-angiogenic factors, where imbalances lead to several diseases including cancer. In addition, both V2 and V3 exhibited remarkable stability toward proteolytic hydrolysis, suggesting that V2 and V3 are promising therapeutic agents for the intervention of disease conditions arising due to angiogenic imbalances and could also be used as novel molecular switching probes to interrogate the mechanism of VEGFR signaling. The findings also further demonstrated the potential of sulfono-γ-AA peptides to mimic the α-helical domain for protein recognition and modulation of protein-protein interactions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

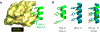
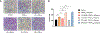
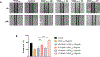




Similar articles
-
Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases.J Biochem. 2013 Jan;153(1):13-9. doi: 10.1093/jb/mvs136. Epub 2012 Nov 21. J Biochem. 2013. PMID: 23172303 Free PMC article. Review.
-
Sulfono-γ-AApeptides as Protein Helical Domain Mimetics to Manipulate the Angiogenesis.Chembiochem. 2022 Nov 18;23(22):e202200298. doi: 10.1002/cbic.202200298. Epub 2022 Sep 12. Chembiochem. 2022. PMID: 36006398 Free PMC article.
-
Eriocalyxin B, a natural diterpenoid, inhibited VEGF-induced angiogenesis and diminished angiogenesis-dependent breast tumor growth by suppressing VEGFR-2 signaling.Oncotarget. 2016 Dec 13;7(50):82820-82835. doi: 10.18632/oncotarget.12652. Oncotarget. 2016. PMID: 27756875 Free PMC article.
-
Xiaotan Sanjie decoction inhibits angiogenesis in gastric cancer through Interleukin-8-linked regulation of the vascular endothelial growth factor pathway.J Ethnopharmacol. 2016 Aug 2;189:230-7. doi: 10.1016/j.jep.2016.05.043. Epub 2016 May 17. J Ethnopharmacol. 2016. PMID: 27224240
-
Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor.Oncologist. 2015 Jun;20(6):660-73. doi: 10.1634/theoncologist.2014-0465. Epub 2015 May 22. Oncologist. 2015. PMID: 26001391 Free PMC article. Review.
Cited by
-
Helical sulfono-γ-AApeptides with predictable functions in protein recognition.RSC Chem Biol. 2022 May 20;3(7):805-814. doi: 10.1039/d2cb00049k. eCollection 2022 Jul 6. RSC Chem Biol. 2022. PMID: 35866163 Free PMC article. Review.
-
Harnessing Aromatic-Histidine Interactions through Synergistic Backbone Extension and Side Chain Modification.Angew Chem Int Ed Engl. 2023 Oct 2;62(40):e202308100. doi: 10.1002/anie.202308100. Epub 2023 Aug 29. Angew Chem Int Ed Engl. 2023. PMID: 37587780 Free PMC article.
-
Promoting implant osseointegration via the osteoblast-selective β-amino acid polymer strategy.Nat Commun. 2025 Aug 5;16(1):7190. doi: 10.1038/s41467-025-58394-1. Nat Commun. 2025. PMID: 40764473 Free PMC article.
-
Exploring the seas for cancer cures: the promise of marine-derived bioactive peptide.Int J Biochem Mol Biol. 2024 Aug 25;15(4):100-106. doi: 10.62347/JGQA3746. eCollection 2024. Int J Biochem Mol Biol. 2024. PMID: 39309615 Free PMC article. Review.
-
Flavonoids with Anti-Angiogenesis Function in Cancer.Molecules. 2024 Mar 31;29(7):1570. doi: 10.3390/molecules29071570. Molecules. 2024. PMID: 38611849 Free PMC article. Review.
References
-
- Sherwood LM; Parris EE; Folkman J Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 1971, 285 (21), 1182–6. - PubMed
-
- Shubik P Vascularization of tumors: a review. J. Cancer Res. Clin. Oncol. 1982, 103 (3), 211–26. - PubMed
-
- Folkman J Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29, 15–18. - PubMed
-
- Carmeliet P; Jain RK Angiogenesis in cancer and other diseases. Nature 2000, 407 (6801), 249–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

