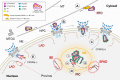
HIV-1 capsid is the key orchestrator of early viral replication
- PMID: 34968390
- PMCID: PMC8717999
- DOI: 10.1371/journal.ppat.1010109
HIV-1 capsid is the key orchestrator of early viral replication
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

