Mechanisms of ketamine and its metabolites as antidepressants
- PMID: 34968492
- PMCID: PMC8883502
- DOI: 10.1016/j.bcp.2021.114892
Mechanisms of ketamine and its metabolites as antidepressants
Abstract
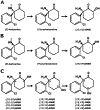
Treating major depression is a medical need that remains unmet by monoaminergic therapeutic strategies that commonly fail to achieve symptom remission. A breakthrough in the treatment of depression was the discovery that the anesthetic (R,S)-ketamine (ketamine), when administered at sub-anesthetic doses, elicits rapid (sometimes within hours) antidepressant effects in humans that are otherwise resistant to monoaminergic-acting therapies. While this finding was revolutionary and led to the FDA approval of (S)-ketamine (esketamine) for use in adults with treatment-resistant depression and suicidal ideation, the mechanisms underlying how ketamine or esketamine elicit their effects are still under active investigation. An emerging view is that metabolism of ketamine may be a crucial step in its mechanism of action, as several metabolites of ketamine have neuroactive effects of their own and may be leveraged as therapeutics. For example, (2R,6R)-hydroxynorketamine (HNK), is readily observed in humans following ketamine treatment and has shown therapeutic potential in preclinical tests of antidepressant efficacy and synaptic potentiation while being devoid of the negative adverse effects of ketamine, including its dissociative properties and abuse potential. We discuss preclinical and clinical studies pertaining to how ketamine and its metabolites produce antidepressant effects. Specifically, we explore effects on glutamate neurotransmission through N-methyl D-aspartate receptors (NMDARs) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), synaptic structural changes via brain derived neurotrophic factor (BDNF) signaling, interactions with opioid receptors, and the enhancement of serotonin, norepinephrine, and dopamine signaling. Strategic targeting of these mechanisms may result in novel rapid-acting antidepressants with fewer undesirable side effects compared to ketamine.
Keywords: (2R,6R)-Hydroxynorketamine; Antidepressant; Depression; Glutamate; Ketamine; NMDAR.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Interest
E.M.H., L.M.R. and M.M. report no conflicts of interest. M.M. has received research funding from AstraZeneca, Redpin Therapeutics, and Attune Neurosciences. T.D.G. is a coauthor on patents and patent applications related to the manufacture, structure, and use of (
Figures

References
-
- Adamson SJ, Sellman JD, Foulds JA, Frampton CM, Deering D, Dunn A, Berks J, Nixon L, & Cape G (2015). A randomized trial of combined citalopram and naltrexone for nonabstinent outpatients with co-occurring alcohol dependence and major depression. J Clin Psychopharmacol, 35(2), 143–149. 10.1097/jcp.0000000000000287 - DOI - PubMed
-
- Ago Y, Tanabe W, Higuchi M, Tsukada S, Tanaka T, Yamaguchi T, Igarashi H, Yokoyama R, Seiriki K, Kasai A, Nakazawa T, Nakagawa S, Hashimoto K, & Hashimoto H (2019). (R)-Ketamine Induces a Greater Increase in Prefrontal 5-HT Release Than (S)-Ketamine and Ketamine Metabolites via an AMPA Receptor-Independent Mechanism. Int J Neuropsychopharmacol, 22(10), 665–674. 10.1093/ijnp/pyz041 - DOI - PMC - PubMed
-
- Aguilar-Valles A, De Gregorio D, Matta-Camacho E, Eslamizade MJ, Khlaifia A, Skaleka A, Lopez-Canul M, Torres-Berrio A, Bermudez S, Rurak GM, Simard S, Salmaso N, Gobbi G, Lacaille JC, & Sonenberg N (2021). Antidepressant actions of ketamine engage cell-specific translation via eIF4E. Nature, 590(7845), 315–319. 10.1038/s41586-020-03047-0 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

