Seroprevalence of IgG antibodies against SARS-CoV-2 in India, March 2020 to August 2021: a systematic review and meta-analysis
- PMID: 34968773
- PMCID: PMC8712428
- DOI: 10.1016/j.ijid.2021.12.353
Seroprevalence of IgG antibodies against SARS-CoV-2 in India, March 2020 to August 2021: a systematic review and meta-analysis
Erratum in
-
Corrigendum to 'Seroprevalence of IgG antibodies against SARS-CoV-2 in India, March 2020-August 2021: a systematic review and meta-analysis' [International Journal of Infectious Diseases, Volume 116 (2022) P59-67].Int J Infect Dis. 2022 Jun;119:119. doi: 10.1016/j.ijid.2022.03.051. Epub 2022 Apr 15. Int J Infect Dis. 2022. PMID: 35436666 Free PMC article. No abstract available.
Abstract
Introduction: India experienced 2 waves of COVID-19 pandemic caused by SARS-CoV-2 and reported the second highest caseload globally. Seroepidemiologic studies were done to track the course of the pandemic. We systematically reviewed and synthesized the seroprevalence of SARS-CoV-2 in the Indian population.
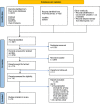
Methods: We included studies reporting seroprevalence of IgG antibodies against SARS-CoV-2 from March 1, 2020 to August 11, 2021 and excluded studies done only among patients with COVID-19 and vaccinated individuals. We searched published databases, preprint servers, and government documents using a combination of keywords and medical subheading (MeSH) terms of "Seroprevalence AND SARS-CoV-2 AND India". We assessed risk of bias using the Newcastle-Ottawa scale, the appraisal tool for cross-sectional studies (AXIS), the Joanna Briggs Institute (JBI) critical appraisal tool, and WHO's statement on the Reporting of Seroepidemiological Studies for SARS-CoV-2 (ROSES-S). We calculated pooled seroprevalence along with 95% Confidence Intervals (CI) during the first (March 2020 to February 2021) and second wave (March to August 2021). We also estimated seroprevalence by selected demographic characteristics.
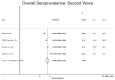
Results: We identified 3821 studies and included 53 studies with 905379 participants after excluding duplicates, screening of titles and abstracts and full-text screening. Of the 53, 20 studies were of good quality. Some of the reviewed studies did not report adequate information on study methods (sampling = 24% (13/53); laboratory = 83% [44/53]). Studies of 'poor' quality had more than one of the following issues: unjustified sample size, nonrepresentative sample, nonclassification of nonrespondents, results unadjusted for demographics and methods insufficiently explained to enable replication. Overall pooled seroprevalence was 20.7% in the first (95% CI = 16.1 to 25.3) and 69.2% (95% CI = 64.5 to 73.8) in the second wave. Seroprevalence did not differ by age in first wave, whereas in the second, it increased with age. Seroprevalence was slightly higher among women in the second wave. In both the waves, the estimate was higher in urban than in rural areas.
Conclusion: Seroprevalence increased by 3-fold between the 2 waves of the pandemic in India. Our review highlights the need for designing and reporting studies using standard protocols.
Keywords: COVID-19; Meta-analysis; SARS-CoV-2; Seroprevalence; Systematic review.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Competing interests Manoj V Murhekar, Tarun Bhatnagar, and Muthusamy Santhosh Kumar were part of the national COVID-19 serosurvey group and coordinated 4 rounds of serosurveys in India. These authors were not involved in the risk of bias assessment.
Figures


References
-
- Alserehi HA, Alqunaibet AM, Al-Tawfiq JA, Alharbi NK, Alshukairi AN, Alanazi KH, et al. Seroprevalence of SARS-CoV-2 (COVID-19) among healthcare workers in Saudi Arabia: comparing case and control hospitals. Diagn Microbiol Infect Dis. 2021;99 doi: 10.1016/j.diagmicrobio.2020.115273. - DOI - PMC - PubMed
-
- bioRxiv.org [internet] 2021. The preprint server for Biology. https://www.biorxiv.org/
-
- Babu GR, Sundaresan R, Athreya S, Akhtar J, Pandey PK, Maroor PS, et al. The burden of active infection and anti-SARS-CoV-2 IgG antibodies in the general population: Results from a statewide survey in Karnataka. India. International Journal of Infectious Diseases. 2021;108:27–36. doi: 10.1101/2020.12.04.20243949. - DOI - PMC - PubMed
-
- Bobrovitz N, Arora RK, Yan T, Rahim H, Duarte N, Boucher E, et al. Lessons from a rapid systematic review of early SARS-CoV-2 serosurveys. MedRxiv 2020:2020.05.10.20097451. 2020 doi: 10.1101/2020.05.10.20097451. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

