Schistosome immunomodulators
- PMID: 34969052
- PMCID: PMC8718004
- DOI: 10.1371/journal.ppat.1010064
Schistosome immunomodulators
Abstract
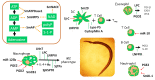
Schistosomes are long lived, intravascular parasitic platyhelminths that infect >200 million people globally. The molecular mechanisms used by these blood flukes to dampen host immune responses are described in this review. Adult worms express a collection of host-interactive tegumental ectoenzymes that can cleave host signaling molecules such as the "alarmin" ATP (cleaved by SmATPDase1), the platelet activator ADP (SmATPDase1, SmNPP5), and can convert AMP into the anti-inflammatory mediator adenosine (SmAP). SmAP can additionally cleave the lipid immunomodulator sphingosine-1-phosphate and the proinflammatory anionic polymer, polyP. In addition, the worms release a barrage of proteins (e.g., SmCB1, SjHSP70, cyclophilin A) that can impinge on immune cell function. Parasite eggs also release their own immunoregulatory proteins (e.g., IPSE/α1, omega1, SmCKBP) as do invasive cercariae (e.g., Sm16, Sj16). Some schistosome glycans (e.g., LNFPIII, LNnT) and lipids (e.g., Lyso-PS, LPC), produced by several life stages, likewise affect immune cell responses. The parasites not only produce eicosanoids (e.g., PGE2, PGD2-that can be anti-inflammatory) but can also induce host cells to release these metabolites. Finally, the worms release extracellular vesicles (EVs) containing microRNAs, and these too have been shown to skew host cell metabolism. Thus, schistosomes employ an array of biomolecules-protein, lipid, glycan, nucleic acid, and more, to bend host biochemistry to their liking. Many of the listed molecules have been individually shown capable of inducing aspects of the polarized Th2 response seen following infection (with the generation of regulatory T cells (Tregs), regulatory B cells (Bregs) and anti-inflammatory, alternatively activated (M2) macrophages). Precisely how host cells integrate the impact of these myriad parasite products following natural infection is not known. Several of the schistosome immunomodulators described here are in development as novel therapeutics against autoimmune, inflammatory, and other, nonparasitic, diseases.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

