Treatment-refractory ulcerative colitis responsive to indigo naturalis
- PMID: 34969665
- PMCID: PMC8718466
- DOI: 10.1136/bmjgast-2021-000813
Treatment-refractory ulcerative colitis responsive to indigo naturalis
Abstract
Background: Indigo naturalis (IN) is an herbal medicine that has been used for ulcerative colitis with an unclear mechanism of action. Indigo and indirubin, its main constituents, are ligands of the aryl hydrocarbon receptor (AhR). We assessed the safety, efficacy, and colon AhR activity of IN given orally to patients with treatment-refractory ulcerative colitis. The role of AhR in IN benefit was further evaluated with an AhR antagonist in a murine colitis model.
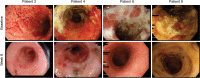
Methods: This open-label, dose-escalation study sequentially treated 11 patients with ulcerative colitis with either IN 500 mg/day or 1.5 g/day for 8 weeks, followed by a 4-week non-treatment period. The primary efficacy endpoint was clinical response at week 8, assessed by total Mayo score. Secondary endpoints included clinical remission, Ulcerative Colitis Endoscopic Index of Severity, quality of life, and colon AhR activity measured by cytochrome P450 1A1 (CYP1A1) RNA expression.
Results: Ten of 11 (91%) patients, including 8/9 (89%) with moderate-to-severe disease, achieved a clinical response. Among these 10 patients, all had failed treatment with 5-aminosalicylic acid, 8 patients with a tumour necrosis factor (TNF)-alpha inhibitor, and 6 patients with TNF-alpha inhibitor and vedolizumab. Five patients were corticosteroid dependent. Clinical response was observed in all five patients who had been recommended for colectomy. Three patients achieved clinical remission. All patients experienced improved endoscopic severity and quality of life. Four weeks after treatment completion, six patients had worsened partial Mayo scores. Four patients progressed to colectomy after study completion. Colon CYP1A1 RNA expression increased 12 557-fold at week 8 among six patients evaluated. No patient discontinued IN due to an adverse event. Concomitant administration of 3-methoxy-4-nitroflavone, an AhR antagonist, in a murine colitis model abrogated the benefit of IN.
Conclusion: IN is a potentially effective therapy for patients with treatment-refractory ulcerative colitis. This benefit is likely through AhR activation.
Trial registration number: NCT02442960.
Keywords: clinical trials; inflammatory bowel disease; ulcerative colitis.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JPS and MGD are employed by and have equity in Azora Therapeutics and are co-inventors on a patent for compositions comprising indigo and/or an indigo derivative. JOLA has equity in Azora Therapeutics and is a co-inventor on a patent for compositions comprising indigo and/or an indigo derivative. LRF has stock in Azora Therapeutics related to advisory activities. BL is currently employed at the University of California, Los Angeles and has stock in Azora Therapeutics related to advisory activities. KTP declares no competing interest during the study, is an employee of Genentech, and shareholder of the Roche Group.
Figures




References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
