Hemochromatosis drives acute lethal intestinal responses to hyperyersiniabactin-producing Yersinia pseudotuberculosis
- PMID: 34969677
- PMCID: PMC8764673
- DOI: 10.1073/pnas.2110166119
Hemochromatosis drives acute lethal intestinal responses to hyperyersiniabactin-producing Yersinia pseudotuberculosis
Abstract
Hemachromatosis (iron-overload) increases host susceptibility to siderophilic bacterial infections that cause serious complications, but the underlying mechanisms remain elusive. The present study demonstrates that oral infection with hyperyersiniabactin (Ybt) producing Yersinia pseudotuberculosis Δfur mutant (termed Δfur) results in severe systemic infection and acute mortality to hemochromatotic mice due to rapid disruption of the intestinal barrier. Transcriptome analysis of Δfur-infected intestine revealed up-regulation in cytokine-cytokine receptor interactions, the complement and coagulation cascade, the NF-κB signaling pathway, and chemokine signaling pathways, and down-regulation in cell-adhesion molecules and Toll-like receptor signaling pathways. Further studies indicate that dysregulated interleukin (IL)-1β signaling triggered in hemachromatotic mice infected with Δfur damages the intestinal barrier by activation of myosin light-chain kinases (MLCK) and excessive neutrophilia. Inhibiting MLCK activity or depleting neutrophil infiltration reduces barrier disruption, largely ameliorates immunopathology, and substantially rescues hemochromatotic mice from lethal Δfur infection. Moreover, early intervention of IL-1β overproduction can completely rescue hemochromatotic mice from the lethal infection.
Keywords: Yersinia pseudotuberculosis; hemochromatosis; hyperinflammation; lethal infection; siderophore.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures

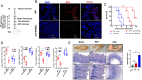

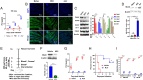

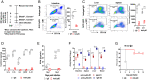
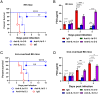
References
-
- Khan F. A., Fisher M. A., Khakoo R. A., Association of hemochromatosis with infectious diseases: Expanding spectrum. Int. J. Infect. Dis. 11, 482–487 (2007). - PubMed
-
- Pietrangelo A., Hereditary hemochromatosis: Pathogenesis, diagnosis, and treatment. Gastroenterology 139, 393–408, 408 e1–2 (2010). - PubMed
-
- Emanuele D., Tuason I., Edwards Q. T., HFE-associated hereditary hemochromatosis: Overview of genetics and clinical implications for nurse practitioners in primary care settings. J. Am. Assoc. Nurse Pract. 26, 113–122 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

