1H detection and dynamic nuclear polarization-enhanced NMR of Aβ1-42 fibrils
- PMID: 34969859
- PMCID: PMC8740738
- DOI: 10.1073/pnas.2114413119
1H detection and dynamic nuclear polarization-enhanced NMR of Aβ1-42 fibrils
Abstract
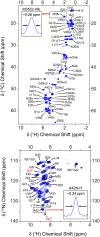
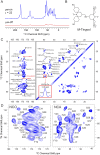
Several publications describing high-resolution structures of amyloid-β (Aβ) and other fibrils have demonstrated that magic-angle spinning (MAS) NMR spectroscopy is an ideal tool for studying amyloids at atomic resolution. Nonetheless, MAS NMR suffers from low sensitivity, requiring relatively large amounts of samples and extensive signal acquisition periods, which in turn limits the questions that can be addressed by atomic-level spectroscopic studies. Here, we show that these drawbacks are removed by utilizing two relatively recent additions to the repertoire of MAS NMR experiments-namely, 1H detection and dynamic nuclear polarization (DNP). We show resolved and sensitive two-dimensional (2D) and three-dimensional (3D) correlations obtained on 13C,15N-enriched, and fully protonated samples of M0Aβ1-42 fibrils by high-field 1H-detected NMR at 23.4 T and 18.8 T, and 13C-detected DNP MAS NMR at 18.8 T. These spectra enable nearly complete resonance assignment of the core of M0Aβ1-42 (K16-A42) using submilligram sample quantities, as well as the detection of numerous unambiguous internuclear proximities defining both the structure of the core and the arrangement of the different monomers. An estimate of the sensitivity of the two approaches indicates that the DNP experiments are currently ∼6.5 times more sensitive than 1H detection. These results suggest that 1H detection and DNP may be the spectroscopic approaches of choice for future studies of Aβ and other amyloid systems.
Keywords: 1H detection; amyloid β1-42; dynamic nuclear polarization; magic-angle spinning.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Chiti F., Dobson C. M., Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 75, 333–366 (2006). - PubMed
-
- Cohen A. S., Amyloidosis. N. Engl. J. Med. 277, 522–530 (1967). - PubMed
-
- Glenner G. G., Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts). N. Engl. J. Med. 302, 1283–1292 (1980). - PubMed
-
- Glenner G. G., Wong C. W., Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 120, 885–890 (1984). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

