Engineering CAR-T cells to activate small-molecule drugs in situ
- PMID: 34969970
- PMCID: PMC9152922
- DOI: 10.1038/s41589-021-00932-1
Engineering CAR-T cells to activate small-molecule drugs in situ
Abstract
Chimeric antigen receptor (CAR)-T cells represent a major breakthrough in cancer therapy, wherein a patient's own T cells are engineered to recognize a tumor antigen, resulting in activation of a local cytotoxic immune response. However, CAR-T cell therapies are currently limited to the treatment of B cell cancers and their effectiveness is hindered by resistance from antigen-negative tumor cells, immunosuppression in the tumor microenvironment, eventual exhaustion of T cell immunologic functions and frequent severe toxicities. To overcome these problems, we have developed a novel class of CAR-T cells engineered to express an enzyme that activates a systemically administered small-molecule prodrug in situ at a tumor site. We show that these synthetic enzyme-armed killer (SEAKER) cells exhibit enhanced anticancer activity with small-molecule prodrugs, both in vitro and in vivo in mouse tumor models. This modular platform enables combined targeting of cellular and small-molecule therapies to treat cancers and potentially a variety of other diseases.
© 2021. The Author(s), under exclusive licence to Springer Nature America, Inc.
Figures



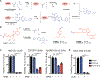
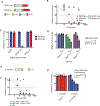




Comment in
-
CAR-T cells SEAK help from enzymes.Nat Chem Biol. 2022 Feb;18(2):122-123. doi: 10.1038/s41589-021-00933-0. Nat Chem Biol. 2022. PMID: 34969971 No abstract available.
-
CAR T Cells Activate Cancer Therapy.Cancer Discov. 2022 Mar 1;12(3):592. doi: 10.1158/2159-8290.CD-NB2022-0005. Cancer Discov. 2022. PMID: 35086924
-
SEAKER cells coordinate cellular immunotherapy with localized chemotherapy.Trends Pharmacol Sci. 2022 Oct;43(10):804-805. doi: 10.1016/j.tips.2022.04.001. Epub 2022 Apr 28. Trends Pharmacol Sci. 2022. PMID: 35491262 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

