Sphingosine Kinase 1 Plays an Important Role in Atorvastatin-Mediated Anti-Inflammatory Effect against Acute Lung Injury
- PMID: 34970075
- PMCID: PMC8714370
- DOI: 10.1155/2021/9247285
Sphingosine Kinase 1 Plays an Important Role in Atorvastatin-Mediated Anti-Inflammatory Effect against Acute Lung Injury
Abstract
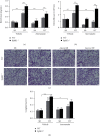
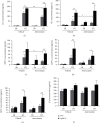
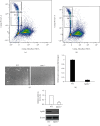
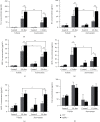
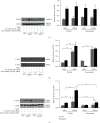
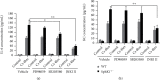
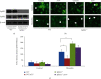
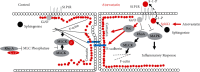
Atorvastatin is a 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA reductase) inhibitor and inhibits cholesterol synthesis. Recently, atorvastatin also showed anti-inflammatory effect in acute lung injury, ameliorating pulmonary gas-blood exchanging function. Sphingosine kinase 1 plays a central role in endothelial (EC) cytoskeleton rearrangement and EC barrier integrity regulation. In this study, the role of sphingosine kinase 1 in atorvastatin anti-inflammatory effect against acute lung injury was investigated. Both wild-type (WT) and SphK1-/- mice were challenged with high tidal volume ventilation (40 ml/kg body weight, 65 breathing/min, 4 hours). The acute lung injury was evaluated and the mechanisms were explored. In WT mice, atorvastatin treatment significantly decreased acute lung injury responding to high tidal volume ventilation (HT), including protein, cellular infiltration, and cytokine releasing; comparing to WT mice, SphK1-/- mice showed significantly worsen pulmonary injuries on HT model. Moreover, the atorvastatin-mediated anti-inflammatory effect was diminished in SphK1-/- mice. To further confirm the role of SphK1 in VILI, we then compared the inflammatory response of endothelial cells that were isolated from WT and SphK1-/- mice to cyclic stretching. Similarly, atorvastatin significantly decreased cytokine generation from WT EC responding to cyclic stretching. Atorvastatin also significantly preserved endothelial junction integrity in WT EC against thrombin challenge. However, the inhibitory effect of atorvastatin on cytokine generation induced by cyclic stretching was abolished on SphK1-/- mice EC. The endothelial junction integrity effects of atorvastatin also diminished on SphK1-/- mouse EC. Signal analysis indicated that atorvastatin inhibited JNK activation induced by cyclic stretch. SphK1 knockout also blocked atorvastatin-mediated VE-cadherin junction enhancement. In summary, by inhibition of MAPK activity and maintenance of EC junction homeostasis, SphK1 plays a critical role in atorvastatin-mediated anti-inflammatory effects in both cellular and in vivo model. This study also offers an insight into mechanical stress-mediated acute lung injury and potential therapy in the future.
Copyright © 2021 Lan Wu et al.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

