Characterizing the Access of Cholinergic Antagonists to Efferent Synapses in the Inner Ear
- PMID: 34970112
- PMCID: PMC8712681
- DOI: 10.3389/fnins.2021.754585
Characterizing the Access of Cholinergic Antagonists to Efferent Synapses in the Inner Ear
Abstract
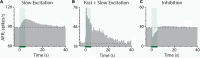
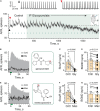
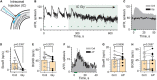
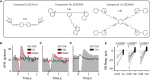
Stimulation of cholinergic efferent neurons innervating the inner ear has profound, well-characterized effects on vestibular and auditory physiology, after activating distinct ACh receptors (AChRs) on afferents and hair cells in peripheral endorgans. Efferent-mediated fast and slow excitation of vestibular afferents are mediated by α4β2*-containing nicotinic AChRs (nAChRs) and muscarinic AChRs (mAChRs), respectively. On the auditory side, efferent-mediated suppression of distortion product otoacoustic emissions (DPOAEs) is mediated by α9α10nAChRs. Previous characterization of these synaptic mechanisms utilized cholinergic drugs, that when systemically administered, also reach the CNS, which may limit their utility in probing efferent function without also considering central effects. Use of peripherally-acting cholinergic drugs with local application strategies may be useful, but this approach has remained relatively unexplored. Using multiple administration routes, we performed a combination of vestibular afferent and DPOAE recordings during efferent stimulation in mouse and turtle to determine whether charged mAChR or α9α10nAChR antagonists, with little CNS entry, can still engage efferent synaptic targets in the inner ear. The charged mAChR antagonists glycopyrrolate and methscopolamine blocked efferent-mediated slow excitation of mouse vestibular afferents following intraperitoneal, middle ear, or direct perilymphatic administration. Both mAChR antagonists were effective when delivered to the middle ear, contralateral to the side of afferent recordings, suggesting they gain vascular access after first entering the perilymphatic compartment. In contrast, charged α9α10nAChR antagonists blocked efferent-mediated suppression of DPOAEs only upon direct perilymphatic application, but failed to reach efferent synapses when systemically administered. These data show that efferent mechanisms are viable targets for further characterizing drug access in the inner ear.
Keywords: DPOAE; auditory efferents; mouse; muscarinic; nicotinic; vestibular efferents.
Copyright © 2021 Lee, Sinha, Henry, Walbaum, Crooks and Holt.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Muscarinic Acetylcholine Receptors and M-Currents Underlie Efferent-Mediated Slow Excitation in Calyx-Bearing Vestibular Afferents.J Neurosci. 2017 Feb 15;37(7):1873-1887. doi: 10.1523/JNEUROSCI.2322-16.2017. Epub 2017 Jan 16. J Neurosci. 2017. PMID: 28093476 Free PMC article.
-
Pharmacologically distinct nicotinic acetylcholine receptors drive efferent-mediated excitation in calyx-bearing vestibular afferents.J Neurosci. 2015 Feb 25;35(8):3625-43. doi: 10.1523/JNEUROSCI.3388-14.2015. J Neurosci. 2015. PMID: 25716861 Free PMC article.
-
Elucidating the role of muscarinic acetylcholine receptor (mAChR) signaling in efferent mediated responses of vestibular afferents in mammals.bioRxiv [Preprint]. 2023 Aug 6:2023.07.31.549902. doi: 10.1101/2023.07.31.549902. bioRxiv. 2023. PMID: 37577578 Free PMC article. Preprint.
-
A review of synaptic mechanisms of vestibular efferent signaling in turtles: extrapolation to efferent actions in mammals.J Vestib Res. 2013;23(3):161-75. doi: 10.3233/VES-130492. J Vestib Res. 2013. PMID: 24177348 Review.
-
Development of the inner ear efferent system across vertebrate species.J Neurobiol. 2002 Nov 5;53(2):228-50. doi: 10.1002/neu.10130. J Neurobiol. 2002. PMID: 12382278 Review.
Cited by
-
Exploring the origins of decreased sound tolerance in tinnitus patients.Front Neurol. 2023 Nov 6;14:1273705. doi: 10.3389/fneur.2023.1273705. eCollection 2023. Front Neurol. 2023. PMID: 38020634 Free PMC article.
References
-
- Bath A. P., Walsh R. M., Bance M. L., Rutka J. A. (1999). Ototoxicity of topical gentamicin preparations. Laryngoscope 109 1088–1093. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

