Study on the Molecular Basis of Huanglian Jiedu Decoction Against Atopic Dermatitis Integrating Chemistry, Biochemistry, and Metabolomics Strategies
- PMID: 34970141
- PMCID: PMC8712871
- DOI: 10.3389/fphar.2021.770524
Study on the Molecular Basis of Huanglian Jiedu Decoction Against Atopic Dermatitis Integrating Chemistry, Biochemistry, and Metabolomics Strategies
Abstract
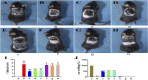
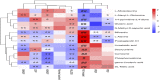
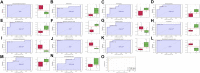
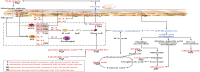
Atopic dermatitis (AD) is a common chronic relapsing skin inflammation, which severely affect the quality of life of patients. Inhibiting itching and enhancing immunity to mitigate scratching are key elements in the fight against AD. Huanglian Jiedu decoction (HLJDD) has multiple pharmacological effects in the treatment of AD. However, the effective ingredients and underlying molecular mechanisms have not yet been fully explored. Thus, this study integrates chemistry, biochemistry, and metabolomics strategies to evaluate the active substance basis of HLJDD against AD. First, HLJDD was split to five fractions (CPF, 40AEF, 90AEF, PEF and WEF) and 72 chemical components were identified. NSD (Non-similarity degree) among the different fractions showed significant chemical differences (>81%). Interleukin IL-13, IL-17A, IL-3, IL-31, IL-33, IL4, IL-5, TSLP, IgE, and histamine in the serum, and IL-4Rα, JAK1, and HRH4 levels in skin, participating in inhibiting itching and regulating immunity signaling, were found to be restored to varying degrees in AD treating with HLJDD and its fractions, especially 40AEF and CPF. Untargeted metabolomics analysis demonstrated that forty metabolites were differential metabolites in plasma between the HLJDD-treated group and the AD group, involving in histidine metabolism, arginine biosynthesis, pyrimidine metabolism, and so on. Further, targeted metabolomics analysis revealed that eleven differential metabolites, associating with physiological and biochemical indices, were significant improved in the HLJDD and its fractions groups. In conclusion, HLJDD exhibited anti-AD effects by inhibiting itching and enhancing immunity, which in turn regulating the levels of relative metabolites, and CPF and 40AEF were considered the most important components of HLJDD.
Keywords: atopic dermatitis (AD); huanglian jiedu decoction; mechanism; metabolomics; molecular basis.
Copyright © 2021 Chen, Chen, Chen, Shen, Jiang and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


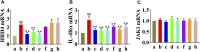





References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

