Evaluation of the Efficacy and Safety of a Compound of Micronized Flavonoids in Combination With Vitamin C and Extracts of Centella asiatica, Vaccinium myrtillus, and Vitis vinifera for the Reduction of Hemorrhoidal Symptoms in Patients With Grade II and III Hemorrhoidal Disease: A Retrospective Real-Life Study
- PMID: 34970145
- PMCID: PMC8712720
- DOI: 10.3389/fphar.2021.773320
Evaluation of the Efficacy and Safety of a Compound of Micronized Flavonoids in Combination With Vitamin C and Extracts of Centella asiatica, Vaccinium myrtillus, and Vitis vinifera for the Reduction of Hemorrhoidal Symptoms in Patients With Grade II and III Hemorrhoidal Disease: A Retrospective Real-Life Study
Abstract
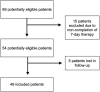
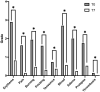
Background and Aim: Several evidences have shown how, in hemorrhoidal disease, phlebotonic flavonoid agents such as quercetin reduce capillary permeability by increasing vascular walls resistance, how rutin and vitamin C have antioxidant properties, and that Centella asiatica has reparative properties towards the connective tissue. A retrospective study was designed in order to evaluate the efficacy and safety of a compound consisting of micronized flavonoids in combination with vitamin C and extracts of C. asiatica, Vaccinium myrtillus, and Vitis vinifera for grade II and III hemorrhoidal disease. Patients and Methods: Data of 49 patients, over 18, who were following a free diet regimen, not on therapy with other anti-hemorrhoid agents, treated with a compound consisting of 450 mg of micronized diosmin, 300 mg of C. asiatica, 270 mg of micronized hesperidin, 200 mg of V. vinifera, 160 mg of vitamin C, 160 mg of V. myrtillus, 140 mg of micronized quercetin, and 130 mg of micronized rutin (1 sachet or 2 tablets a day) for 7 days were collected. Hemorrhoid grade according to Goligher's scale together with anorectal symptoms (edema, prolapse, itching, thrombosis, burning, pain, tenesmus, and bleeding) both before treatment (T0) and after 7 days of therapy (T7) were collected. Primary outcomes were the reduction of at least one degree of hemorrhoids according to Goligher's scale assessed by proctological examination and compound safety. The secondary outcome was the reduction of anorectal symptoms assessed by questionnaires administered to patients. Results: Forty-four patients (89.8%) presented a reduction in hemorrhoidal grade of at least one grade (p < 0.001). No adverse events with the use of the compound were noted. A significant reduction was observed in all anorectal symptoms evaluated (p < 0.05). No predictors of response to the compound were identified among the clinical and demographic variables collected. Conclusion: The compound analyzed was effective and safe for patients with grade II and III hemorrhoidal disease according to Goligher's scale.
Keywords: Centella asiatica; Vaccinium myrtillus; Vitis vinifera; flavonoids; hemorrhoidal disease; vitamin C.
Copyright © 2021 Gravina, Pellegrino, Facchiano, Palladino, Loguercio and Federico.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Flavonoid mixture (diosmin, troxerutin, rutin, hesperidin, quercetin) in the treatment of I-III degree hemorroidal disease: a double-blind multicenter prospective comparative study.Int J Colorectal Dis. 2018 Nov;33(11):1595-1600. doi: 10.1007/s00384-018-3102-y. Epub 2018 Jun 22. Int J Colorectal Dis. 2018. PMID: 29934701 Clinical Trial.
-
[Treatment of acute hemorrhoids according to the results of a multicenter observational study].Khirurgiia (Mosk). 2025;(3):112-123. doi: 10.17116/hirurgia2025031112. Khirurgiia (Mosk). 2025. PMID: 40103253 Russian.
-
Conservative Treatment of Hemorrhoids: Results of an Observational Multicenter Study.Adv Ther. 2018 Nov;35(11):1979-1992. doi: 10.1007/s12325-018-0794-x. Epub 2018 Oct 1. Adv Ther. 2018. PMID: 30276625 Free PMC article.
-
Transanal hemorrhoidal dearterialization (THD) for hemorrhoidal disease: a single-center study on 1000 consecutive cases and a review of the literature.Tech Coloproctol. 2017 Dec;21(12):953-962. doi: 10.1007/s10151-017-1726-5. Epub 2017 Nov 24. Tech Coloproctol. 2017. PMID: 29170839 Free PMC article. Review.
-
Efficacy and safety of micronized purified flavonoid fractions for the treatment of postoperative hemorrhoid complications: A systematic review and meta-analysis.Phytomedicine. 2022 Sep;104:154244. doi: 10.1016/j.phymed.2022.154244. Epub 2022 Jun 17. Phytomedicine. 2022. PMID: 35752073
Cited by
-
Correlation Between Poor Defecation Habits and Postoperative Hemorrhoid Recurrence.Front Surg. 2022 Jun 17;9:930215. doi: 10.3389/fsurg.2022.930215. eCollection 2022. Front Surg. 2022. PMID: 35784912 Free PMC article.
-
Shifting Paradigms in Hemorrhoid Management: The Emergence and Impact of Cap-Assisted Endoscopic Sclerotherapy.J Clin Med. 2024 Nov 29;13(23):7284. doi: 10.3390/jcm13237284. J Clin Med. 2024. PMID: 39685741 Free PMC article. Review.
-
The Potential Mechanism of Kushen Decoction in Treating Haemorrhoids: An Integration of Network Pharmacology, Molecular Docking and Molecular Dynamics Simulation.IET Syst Biol. 2025 Jan-Dec;19(1):e70029. doi: 10.1049/syb2.70029. IET Syst Biol. 2025. PMID: 40694626 Free PMC article.
References
-
- Aguilar Peralta G. R., Arévalo Gardoqui J., Llamas Macías F. J., Navarro Ceja V. H., Mendoza Cisneros S. A., Martínez Macías C. G. (2007). Clinical and Capillaroscopic Evaluation in the Treatment of Chronic Venous Insufficiency with Ruscus Aculeatus, Hesperidin Methylchalcone and Ascorbic Acid in Venous Insufficiency Treatment of Ambulatory Patients. Int. Angiol 26, 378–384. - PubMed
-
- Beck D. E., Steele S. R., Wexner S. D. (1998). Fundamentals of Anorectal Surgery. 2nd Edition. WB Saunders.
-
- Beck D. E. (2011). The ASCRS Textbook of Colon and Rectal Surgery. 2nd Edition.
-
- Bogucka-Kocka A., Woźniak M., Feldo M., Kockic J., Szewczyk K. (2013). Diosmin--isolation Techniques, Determination in Plant Material and Pharmaceutical Formulations, and Clinical Use. Nat. Prod. Commun. 8, 545–550. - PubMed
LinkOut - more resources
Full Text Sources

