Taxonomic Structure of Rhizosphere Bacterial Communities and Its Association With the Accumulation of Alkaloidal Metabolites in Sophora flavescens
- PMID: 34970241
- PMCID: PMC8712762
- DOI: 10.3389/fmicb.2021.781316
Taxonomic Structure of Rhizosphere Bacterial Communities and Its Association With the Accumulation of Alkaloidal Metabolites in Sophora flavescens
Abstract
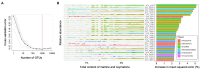
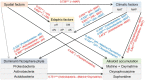
Plant secondary metabolites (SMs) play a crucial role in plant defense against pathogens and adaptation to environmental stresses, some of which are produced from medicinal plants and are the material basis of clinical efficacy and vital indicators for quality evaluation of corresponding medicinal materials. The influence of plant microbiota on plant nutrient uptake, production, and stress tolerance has been revealed, but the associations between plant microbiota and the accumulation of SMs in medicinal plants remain largely unknown. Plant SMs can vary among individuals, which could be partly ascribed to the shift in microbial community associated with the plant host. In the present study, we sampled fine roots and rhizosphere soils of Sophora flavescens grown in four well-separated cities/counties in China and determined the taxonomic composition of rhizosphere bacterial communities using Illumina 16S amplicon sequencing. In addition, the association of the rhizosphere bacterial microbiota with the accumulation of alkaloids in the roots of S. flavescens was analyzed. The results showed that S. flavescens hosted distinct bacterial communities in the rhizosphere across geographic locations and plant ages, also indicating that geographic location was a larger source of variation than plant age. Moreover, redundancy analysis revealed that spatial, climatic (mean annual temperature and precipitation), and edaphic factors (pH and available N and P) were the key drivers that shape the rhizosphere bacterial communities. Furthermore, the results of the Mantel test demonstrated that the rhizosphere bacterial microbiota was remarkably correlated with the contents of oxymatrine, sophoridine, and matrine + oxymatrine in roots. Specific taxa belonging to Actinobacteria and Chloroflexi were identified as potential beneficial bacteria associated with the total accumulation of matrine and oxymatrine by a random forest machine learning algorithm. Finally, the structural equation modeling indicated that the Actinobacteria phylum had a direct effect on the total accumulation of matrine and oxymatrine. The present study addresses the association between the rhizosphere bacterial communities and the accumulation of alkaloids in the medicinal plant S. flavescens. Our findings may provide a basis for the quality improvement and sustainable utilization of this medicinal plant thorough rhizosphere microbiota manipulation.
Keywords: alkaloids; medicinal plants; plant bacterial community; rhizosphere; secondary metabolites.
Copyright © 2021 Chen, Li, Chang, Ren, Zhou and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Bao S. (2000). Soil and Agricultural Chemistry Analysis. Beijing: China Agriculture Press.
-
- Borcard D., Gillet F., Legendre P. (2011). Numerical Ecology With R. New York: Springer Science and Business Media.
LinkOut - more resources
Full Text Sources

