Galactose-Binding C-Type Lectin Promotes Cellular Aggregation of Coelomocytes in Sea Cucumber
- PMID: 34970266
- PMCID: PMC8713890
- DOI: 10.3389/fimmu.2021.783798
Galactose-Binding C-Type Lectin Promotes Cellular Aggregation of Coelomocytes in Sea Cucumber
Abstract
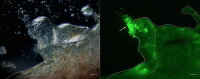
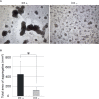
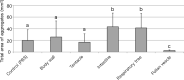
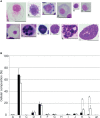
Echinoderms have a large coelomic cavity containing coelomocytes. When the coelomic fluid is removed from the cavity, the cells aggregate immediately. We found that a fraction or an extract of the intestine of the sea cucumber, Apostichopus japonicus, markedly accelerated cellular movement and aggregation on a glass slide, and this effect was clearly inhibited by galactose. We successfully purified the aggregation-promoting factor, a 16 kDa protein, from the intestine. TOF-MS analysis followed by de novo sequencing revealed that the protein is a C-type lectin. RNA-seq data and cDNA cloning demonstrated the factor to be a novel lectin, named AjGBCL, consisting of 158 aa residues in the mature form. Microscopic observation revealed that most of the aggregating cells moved toward aggregates and not to an intestinal fragment, suggesting that AjGBCL is not a chemoattractant but a cellular aggregation-inducing factor that may induce aggregates to release chemoattractant. We report, for the first time, an endogenous molecule that promotes coelomocyte aggregation in echinoderms.
Keywords: cell migration; cellular aggregation; coelomocytes; lectin; sea cucumber.
Copyright © 2021 Taguchi, Tanaka, Tsutsui and Nakamura.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Ramirez-Gomez FJ, Garcia-Arraras JE. Echinoderm Immunity. ISJ Invertebr Surviv J (2010) 7:211–20.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

