Alpha-2-Macroglobulin in Inflammation, Immunity and Infections
- PMID: 34970276
- PMCID: PMC8712716
- DOI: 10.3389/fimmu.2021.803244
Alpha-2-Macroglobulin in Inflammation, Immunity and Infections
Abstract
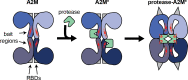
Alpha-2-macroglobulin is an extracellular macromolecule mainly known for its role as a broad-spectrum protease inhibitor. By presenting itself as an optimal substrate for endopeptidases of all catalytic types, alpha-2-macroglobulin lures active proteases into its molecular cage and subsequently 'flags' their complex for elimination. In addition to its role as a regulator of extracellular proteolysis, alpha-2-macroglobulin also has other functions such as switching proteolysis towards small substrates, facilitating cell migration and the binding of cytokines, growth factors and damaged extracellular proteins. These functions appear particularly important in the context of immune-cell function. In this review manuscript, we provide an overview of all functions of alpha-2-macroglobulin and place these in the context of inflammation, immunity and infections.
Keywords: alpha-2-macroglobulin; immunity; infections; inflammation; macrophages; neutrophils; proteolysis.
Copyright © 2021 Vandooren and Itoh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

