Plant Proteoforms Under Environmental Stress: Functional Proteins Arising From a Single Gene
- PMID: 34970290
- PMCID: PMC8712444
- DOI: 10.3389/fpls.2021.793113
Plant Proteoforms Under Environmental Stress: Functional Proteins Arising From a Single Gene
Abstract
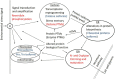
Proteins are directly involved in plant phenotypic response to ever changing environmental conditions. The ability to produce multiple mature functional proteins, i.e., proteoforms, from a single gene sequence represents an efficient tool ensuring the diversification of protein biological functions underlying the diversity of plant phenotypic responses to environmental stresses. Basically, two major kinds of proteoforms can be distinguished: protein isoforms, i.e., alterations at protein sequence level arising from posttranscriptional modifications of a single pre-mRNA by alternative splicing or editing, and protein posttranslational modifications (PTMs), i.e., enzymatically catalyzed or spontaneous modifications of certain amino acid residues resulting in altered biological functions (or loss of biological functions, such as in non-functional proteins that raised as a product of spontaneous protein modification by reactive molecular species, RMS). Modulation of protein final sequences resulting in different protein isoforms as well as modulation of chemical properties of key amino acid residues by different PTMs (such as phosphorylation, N- and O-glycosylation, methylation, acylation, S-glutathionylation, ubiquitinylation, sumoylation, and modifications by RMS), thus, represents an efficient means to ensure the flexible modulation of protein biological functions in response to ever changing environmental conditions. The aim of this review is to provide a basic overview of the structural and functional diversity of proteoforms derived from a single gene in the context of plant evolutional adaptations underlying plant responses to the variability of environmental stresses, i.e., adverse cues mobilizing plant adaptive mechanisms to diminish their harmful effects.
Keywords: biological functions; crops; environmental stresses; protein isoforms; protein posttranslational modifications (PTMs); protein-protein interactions.
Copyright © 2021 Kosová, Vítámvás, Prášil, Klíma and Renaut.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Ajadi A. A., Cisse A., Ahmad S., Wang Y., Shu Y., Li S., et al. (2020). Protein phosphorylation and phosphoproteome: an overview. Rice Sci. 27 184–200. 10.1016/j.rsci.2020.04.003 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

