COVID-19-related adaptations to the implementation and evaluation of a clinic-based intervention designed to improve opioid safety
- PMID: 34970321
- PMCID: PMC8687093
- DOI: 10.7573/dic.2021-7-5
COVID-19-related adaptations to the implementation and evaluation of a clinic-based intervention designed to improve opioid safety
Abstract
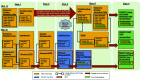
The United States faces an opioid crisis with an unprecedented and increasing death rate from opioid overdose. Successfully reducing the rates of opioid use disorder (OUD) and overdose will require the engagement of frontline clinicians to prescribe opioids more safely and to build their capacity to treat patients with OUD using evidence-based approaches. The COVID-19 pandemic has created significant challenges for patients, clinicians and health systems and has been associated with increasing risks of overdoses and deaths. Herein, we review a multidisciplinary project designed to implement and evaluate clinic-based interventions in Oregon, USA, to improve pain management, opioid prescribing and treatment of OUD. The intervention, called Improving PaIn aNd OPiOId MaNagemenT in Primary Care (PINPOINT), combines practice facilitation, academic detailing and education through the Oregon ECHO Network. Implementation of PINPOINT has occurred across the Oregon Rural Practice-based Research Network and has involved 49 clinic sites to date. To evaluate the impact of the intervention, the research team created the Provider Results of Opioid Management and Prescribing Training (PROMPT), a dataset that links information from the state prescription drug monitoring program, all-payer claims database, emergency medical services, vital records and substance use disorder treatment system. The PROMPT dataset will allow evaluation of the impact of the intervention at both the clinician and clinic levels. Due to the constraints of the COVID-19 pandemic, elements of both implementation and evaluation required significant adaptations to continue to meet the original project goals.
Keywords: academic detailing; all-payer claims database; opioid safety; practice facilitation; prescription drug monitoring programs.
Copyright © 2021 Morgan AR, Hendricks MA, El Ibrahimi S, Hallvik SE, Hatch B, Dickinson C, Wright D, Fischer MA.
Conflict of interest statement
Disclosure and potential conflicts of interest: MAF serves as a clinical consultant for Alosa Health, an educational non-profit that provides academic detailing services, and directs the National Resource Center for Academic Detailing, a program within the Division of Pharmacoepidemiology and Pharmacoeconomics. NaRCAD is funded by grants from AHRQ (R18HS026177 and R13HS026829) and contracts from the CDC and NACCHO. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2021/10/dic.2021-7-5-COI.pdf
Figures
References
Publication types
LinkOut - more resources
Full Text Sources