High Circulating Sonic Hedgehog Protein Is Associated With Poor Outcome in EGFR-Mutated Advanced NSCLC Treated With Tyrosine Kinase Inhibitors
- PMID: 34970481
- PMCID: PMC8712335
- DOI: 10.3389/fonc.2021.747692
High Circulating Sonic Hedgehog Protein Is Associated With Poor Outcome in EGFR-Mutated Advanced NSCLC Treated With Tyrosine Kinase Inhibitors
Erratum in
-
Corrigendum: High Circulating Sonic Hedgehog Protein Is Associated With Poor Outcome in EGFR-Mutated Advanced NSCLC Treated With Tyrosine Kinase Inhibitors.Front Oncol. 2022 Feb 9;12:852540. doi: 10.3389/fonc.2022.852540. eCollection 2022. Front Oncol. 2022. PMID: 35223530 Free PMC article.
Abstract
Introduction: Growing preclinical evidence has suggested that the Sonic hedgehog (Shh) pathway is involved in resistance to tyrosine kinase inhibitor (TKI) therapy for EGFR-mutated (EGFRm) non-small cell lung cancer (NSCLC). However, little is known concerning the prognostic value of this pathway in this context.
Materials and methods: We investigated the relationship between plasma levels of Shh and EGFRm NSCLC patients' outcome with EGFR TKIs. We included 74 consecutive patients from two institutions with EGFRm advanced NSCLC treated by EGFR TKI as first-line therapy. Plasma samples were collected longitudinally for each patient and were analyzed for the expression of Shh using an ELISA assay. The activation of the Shh-Gli1 pathway was assessed through immunohistochemistry (IHC) of Gli1 and RT-qPCR analysis of the transcripts of Gli1 target genes in 14 available tumor biopsies collected at diagnosis (baseline).
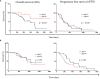
Results: Among the 74 patients, only 61 had baseline (diagnosis) plasma samples, while only 49 patients had plasma samples at the first evaluation. Shh protein was detectable in all samples at diagnosis (n = 61, mean = 1,041.2 ± 252.5 pg/ml). Among the 14 available tumor biopsies, nuclear expression of Gli1 was observed in 57.1% (8/14) of patients' biopsies. Shh was significantly (p < 0.05) enriched in youth (age < 68), male, nonsmokers, patients with a PS > 1, and patients presenting more than 2 metastatic sites and L858R mutation. Higher levels of Shh correlated with poor objective response to TKI, shorter progression-free survival (PFS), and T790M-independent mechanism of resistance. In addition, the rise of plasma Shh levels along the treatment was associated with the emergence of drug resistance in patients presenting an initial good therapy response.
Conclusion: These data support that higher levels of plasma Shh at diagnosis and increased levels of Shh along the course of the disease are related to the emergence of TKI resistance and poor outcome for EGFR-TKI therapy, suggesting that Shh levels could stand both as a prognostic and as a resistance biomarker for the management of EGFR-mutated NSCLC patients treated with EGFR-TKI.
Keywords: Sonic Hedgehog (Shh); biomarker; epidermal growth factor receptor (EGFR); non-small cell lung cancer (NSCLC); tyrosine kinase inhibitor (TKI).
Copyright © 2021 Takam Kamga, Swalduz, Costantini, Julié, Emile, Pérol, Avrillon, Ortiz-Cuaran, de Saintigny and Leprieur.
Conflict of interest statement
EGL: AstraZeneca (honoraria, advisory board, and research grant), Bristol-Myers-Squibb (honoraria, advisory board, and research grant), MSD (honoraria and advisory board); J-FE: Bristol-Myers-Squibb (advisory board); SO-C and PS: AstraZeneca (research grant). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Zhou C, Wu Y-L, Chen G, Feng J, Liu X-Q, Wang C, et al. . Erlotinib Versus Chemotherapy as First-Line Treatment for Patients With Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer (OPTIMAL, CTONG-0802): A Multicentre, Open-Label, Randomised, Phase 3 Study. Lancet Oncol (2011) 12:735–42. doi: 10.1016/S1470-2045(11)70184-X - DOI - PubMed
-
- Wu Y-L, Zhou C, Liam C-K, Wu G, Liu X, Zhong Z, et al. . First-Line Erlotinib Versus Gemcitabine/Cisplatin in Patients With Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer: Analyses From the Phase III, Randomized, Open-Label, ENSURE Study†. Ann Oncol (2015) 26:1883–9. doi: 10.1093/annonc/mdv270 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

