LncRNA FOXP4-AS1 Promotes the Progression of Esophageal Squamous Cell Carcinoma by Interacting With MLL2/H3K4me3 to Upregulate FOXP4
- PMID: 34970490
- PMCID: PMC8712759
- DOI: 10.3389/fonc.2021.773864
LncRNA FOXP4-AS1 Promotes the Progression of Esophageal Squamous Cell Carcinoma by Interacting With MLL2/H3K4me3 to Upregulate FOXP4
Erratum in
-
Corrigendum: LncRNA FOXP4-AS1 promotes the progression of esophageal squamous cell carcinoma by interacting with MLL2/H3K4me3 to upregulate FOXP4.Front Oncol. 2022 Oct 14;12:1041732. doi: 10.3389/fonc.2022.1041732. eCollection 2022. Front Oncol. 2022. PMID: 36313704 Free PMC article.
Abstract
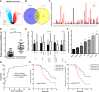
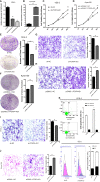
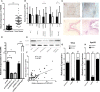
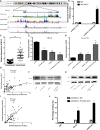
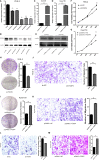
Malignant tumors are a grave threat to human health. Esophageal squamous cell carcinoma (ESCC) is a common gastrointestinal malignant tumor. China has a high incidence of ESCC, and its morbidity and mortality are higher than the global average. Increasingly, studies have shown that long noncoding RNAs (lncRNAs) play a vital function in the occurrence and development of tumors. Although the biological function of FOXP4-AS1 has been demonstrated in various tumors, the potential molecular mechanism of FOXP4-AS1 in ESCC is still poorly understood. The expression of FOXP4 and FOXP4-AS1 was detected in ESCC by quantitative real-time PCR (qRT-PCR) or SP immunohistochemistry (IHC). shRNA was used to silence gene expression. Apoptosis, cell cycle, MTS, colony formation, invasion and migration assays were employed to explore the biological functions of FOXP4 and FOXP4-AS1. The potential molecular mechanism of FOXP4-AS1 in ESCC was determined by dual-luciferase reporter, RNA immunoprecipitation (RIP) and chromatin immunoprecipitation (ChIP). Here, we demonstrated that FOXP4-AS1 was significantly increased in ESCC tissues and cell lines, associated with lymph node metastasis and TNM staging. Cell function experiments showed that FOXP4-AS1 promoted the proliferation, invasion and migration ability of ESCC cells. The expression of FOXP4-AS1 and FOXP4 in ESCC tissues was positively correlated. Further research found that FOXP4-AS1, upregulated in ESCC, promotes FOXP4 expression by enriching MLL2 and H3K4me3 in the FOXP4 promoter through a "molecular scaffold". Moreover, FOXP4, a transcription factor of β-catenin, promotes the transcription of β-catenin and ultimately leads to the malignant progression of ESCC. Finally, FOXP4-AS1 may be a new therapeutic target for ESCC.
Keywords: ESCC; FOXP4; FOXP4-AS1; MLL2; long noncoding RNA.
Copyright © 2021 Niu, Wang, Li, Guo, Guo and Dong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

