FOXO Transcription Factors Are Required for Normal Somatotrope Function and Growth
- PMID: 34971379
- PMCID: PMC8782608
- DOI: 10.1210/endocr/bqab263
FOXO Transcription Factors Are Required for Normal Somatotrope Function and Growth
Abstract
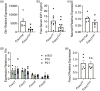
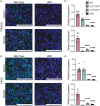
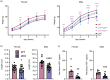
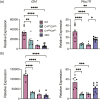
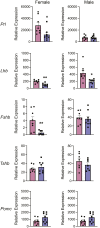
Understanding the molecular mechanisms underlying pituitary organogenesis and function is essential for improving therapeutics and molecular diagnoses for hypopituitarism. We previously found that deletion of the forkhead factor, Foxo1, in the pituitary gland early in development delays somatotrope differentiation. While these mice grow normally, they have reduced growth hormone expression and free serum insulin-like growth factor-1 (IGF1) levels, suggesting a defect in somatotrope function. FOXO factors show functional redundancy in other tissues, so we deleted both Foxo1 and its closely related family member, Foxo3, from the primordial pituitary. We find that this results in a significant reduction in growth. Consistent with this, male and female mice in which both genes have been deleted in the pituitary gland (dKO) exhibit reduced pituitary growth hormone expression and serum IGF1 levels. Expression of the somatotrope differentiation factor, Neurod4, is reduced in these mice. This suggests a mechanism underlying proper somatotrope function is the regulation of Neurod4 expression by FOXO factors. Additionally, dKO mice have reduced Lhb expression and females also have reduced Fshb and Prl expression. These studies reveal FOXO transcription factors as important regulators of pituitary gland function.
Keywords: FOXO1; FOXO3; forkhead; growth hormone; pituitary.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures





References
-
- Li S, Crenshaw EB 3rd, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990;347(6293):528-533. - PubMed
-
- Ando M, Goto M, Hojo M, et al. . The proneural bHLH genes Mash1, Math3 and NeuroD are required for pituitary development. J Mol Endocrinol. 2018;61(3):127-138. - PubMed
-
- Mayo KE, Miller T, DeAlmeida V, Godfrey P, Zheng J, Cunha SR. Regulation of the pituitary somatotroph cell by GHRH and its receptor. Recent Prog Horm Res. 2000;55:237-266; discussion 266-237. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

